Authors:
Pilar Cabanas-Grandío1*, Javier García-Seara1, Francisco Gude2, José Luis Martínez-Sande1, Xesus Alberte Fernández-López1, Felipe Bisbal3, Emad Abu-Assi1 and José Ramón González-Juanatey1
1Cardiology Department, University Clinical Hospital of Santiago de Compostela, Santiago de Compostela, Spain
2Epidemiology Department, University Clinical Hospital of Santiago de Compostela, Santiago de Compostela, Spain
3Cardiology Department; Thorax Institute, Hospital Clínic, Barcelona, Spain
Received: June 27, 2014; Accepted: August 01, 2014; Published: August 04, 2014
Pilar Cabanas-Grandío, Cardiology Department, University Clinical Hospital of Santiago de Compostela, 15706, Santiago de Compostela, Spain, Tel: +34 981950793; Fax: +34 981950534; Email: [email protected]
Cabanas-Grandío P, García-Seara J, Gude F, Martínez-Sande JL, Fernández-López XA, et al. (2014) Echocardiographic Biatrial Remodelling and Diastolic Function Assessment in Long-Term Follow-Up after Typical Atrial Flutter Ablation. J Cardiovasc Med Cardiol 1(1): 011-016. 10.17352/2455-2976.000003
© 2014 Cabanas-Grandío P. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Typical atrial flutter; Ablation; Atrial remodelling; Diastolic function
AAD: Antiarrhythmic Drugs; AF: Atrial Fibrillation; AFl: Atrial Flutter; COPD: Chronic Obstructive Pulmonary Disease; CTI: Cavotricuspid Isthmus; DTE: Deceleration Time of the E Wave; IVRT: Isovolumetric Relaxation Time; LA: Left Atrium; LVEDD: Left Ventricular End Diastolic Diameter; LVEF: Left Ventricular Ejection Fraction; LVESD: Left Ventricular End Systolic Diameter; OAC: Oral Anticoagulation; RA: Right Atrium; RAEDA: Right Atrium End Diastolic Area; RAESA: Right Atrium End Systolic Area; RACF: Right Atrium Contraction Fraction
Background: A reverse left atrial (LA) remodelling after atrial fibrillation (AF) ablation has been reported and a relationship between diastolic function and AF is well known. However, there is little information about atrial remodelling and diastolic function after cavotricuspid isthmus (CTI) ablation. We aimed to evaluate long-term biatrial remodelling and diastolic function in patients undergoing CTI ablation.
Methods: A transthoracic echocardiography was performed at baseline and at long-term follow-up (6.3 ± 0.5 years) in a total of 39 patients who underwent AFl ablation. Right atrial end diastolic areas (RAEDA) and end systolic areas (RAESA), right atrial contraction fraction (RACF), mitral A wave velocity, E/A rate and LA diameter were measured. They were compared using the Wilcoxon rank sum test.
Results: Mean (SD) age was 64 (10) years, 82% male, 49% hypertension and 44% prior AF episodes. Basal RAEDA and RAESA were higher than at follow-up: median (IQR) of 24.6 cm2 (19.8-28.2) vs. 20.0 cm2 (16.0-25.0), p = 0.017 and 17.4 cm2 (13.0-19.3) vs. 12.0 cm2 (9.8-17.0), p = 0.001, respectively. RACF was higher at follow-up: 0.41 (0.35-0.45) vs. 0.31 (0.19-0.37), p = 0.001. Basal mitral A wave velocity was lower than at follow-up: 0.51 (0.4-0.6) vs. 0.78 (0.7-0.9), p =0.001 and E/A rate was higher 1.9 (1.2-3.1) vs. 0.9 (0.7-1.1), p = 0.001. LA diameter at baseline was 44.8 mm (39.3-50.7) vs. 46 mm (41.5-51.5) at follow-up, p <0.001.
Conclusion: AFl ablation led to reverse remodelling of the right atrium and improved diastolic dysfunction parameters in the long-term follow-up.
Introduction
Atrial fibrillation (AF) is the most common arrhythmia seen in clinical practice and it is well-known that there is a relationship between AF and diastolic function [1]. It is also known that LA function is recovered after cardioversion to sinus rhythm as well as after AF ablation, and a reverse LA remodelling after AF ablation has also been reported [2-5].
AF and atrial flutter (AFl) are inter-related [6]. It may be that the relationship between diastolic function and AFl is as with AF, and limited studies suggest that AFl ablation also favours LA function recovery, as occurs after AF ablation [7]. However, there is no information about right atrial (RA) and LA anatomical remodelling in the long term follow-up after AFl ablation. Our objective was to investigate echocardiographic biatrial remodelling and diastolic function in the long-term follow-up after cavotricuspid isthmus (CTI) ablation.
Methods
Study population
We included 39 patients who had undergone typical AFl ablation between January 2003 and May 2005 in which a complete echocardiographic study was performed at baseline (first 6 hours after ablation procedure) and at follow-up (mean ± SD of 6.3 ± 0.5 years) to assess echocardiographic remodelling.
All patients included were over 18 years, with at least one episode of documented AFl in the previous 6 months, and demonstrated a CTI-dependent AFl during electrophysiological study, or CTI permeability with an electrocardiogram suggestive of typical AFl, in cases where the procedure was performed in sinus rhythm.
Ablation procedure
Oral anticoagulation was discontinued 2 days before the electrophysiology study and treatment with low-molecular-weight heparin was initiated if the international normalized ratio was <1.5. A standard quadripolar catheter (Usci-Bard Inc.) was used to map the His bundle region, a decapolar catheter (Usci-Bard Inc.) to map the coronary sinus, and a duodecapolar Halo XP catheter (Cordis-Webster Inc.) to map the activation of the anterolateral wall of the RA. Radiofrequency energy was applied for a period of 60 seconds at each point. CTI dependency was confirmed by entrainment when the rhythm at the beginning of the electrophysiology study was AFl or when this was induced in the laboratory. If the patient was in sinus rhythm, bidirectional CTI permeability was confirmed prior to ablation. The aim of the procedure was to achieve bidirectional CTI conduction block. Bidirectional block was defined through the activation sequence of the electrograms in the RA, the bundle of His, and the coronary sinus stimulating at a cycle length of 600 ms from the coronary sinus and from the lower lateral wall of the RA. The persistence of bidirectional block was confirmed 20 min after completion of the procedure.
Echocardiographic study
Echocardiographic studies were performed using a Sequoia C 256 (Siemens®) and a Vivid 7 Dimension (General Electric®), during the first six hours after the ablation procedure and at long-term follow-up. All studies were analyzed by two experienced cardiologists.
A complete study protocol was performed to evaluate all cardiac chambers and to assess atrial remodeling.
Cardiac chamber dimensions were evaluated in parasternal and apical views according to recommendations of the European and American Society of Echocardiography [8,9].
LA anteroposterior diameter was assessed using M-mode in the parasternal short axis (level of large vessels) and its area was assessed using atrial planimetry in the apical four-chamber view during ventricular systole (maximum LA area).
RA was studied in the apical four-chamber view, measuring the end diastolic area (RAEDA) (maximum area that occurs at the end of ventricular systole) and the end systolic area (RAESA) (minimum area that occurs at ventricular diastole). RA contraction fraction (RACF) was calculated with the formula: RAEDA - RAESA / RAEDA.
The mitral A wave velocity (m/s), the mitral E wave velocity (m/s), and the E/A ratio were obtained with pulsed Doppler, to assess the LA contribution to the left ventricular filling [10], and to study the left ventricular diastolic function the mitral filling pattern was also used. A normal mitral filling pattern was defined as when E/A ratio was around 1.2, a deceleration time of the E wave (DTE) was between 160 and 200 ms and an isovolumetric relaxation time (IVRT) between 70-100 ms. A mitral filling pattern type I or type I diastolic dysfunction was defined as when the E/A <0.8, DTE > 200 ms and IVRT > 100 ms. Mitral filling pattern type II or type II diastolic dysfunction (pseudonormal pattern) was defined when E/A was 1-1.5 (with Valsalva maneuvers <1), DTE 160-200 ms and IVRT < 90 ms. Mitral filling pattern type III or type III diastolic dysfunction (restrictive pattern) was defined if the E/A ratio was >1.5, mostly was > 2, DTE <160 ms and IVRT <60 ms. Tissue Doppler was used on the posterolateral mitral annulus to distinguish filling pattern type II (E ‘/ A' <1, E ‘<8.5 cm/s) from normal filling pattern. The pattern in which there was only mitral E wave, due to AF, was defined as lone mitral E wave.
Statistical analysis
Quantitative variables were expressed as mean and standard deviation (SD) or median and interquartile range (IQR) according to normality assumption or not. Qualitative variables were expressed as frequencies and percentages.
The Wilcoxon rank sum test was used to compare the values of echocardiographic parameters at baseline and at follow-up.
All analyses were performed using SPSS Statistics 17.0 (SPSS Inc., Chicago, IL, USA).
Ethical considerations
All patients were informed and gave their informed consent. Our community ethical committee approved the development of this study.
Results
Baseline characteristics
Baseline characteristics are summarized in table 1. Mean age was 64 ± 10 years. History of AF was present in 17 (44%) patients, 46% of patients were on antiarrhythmic drugs (AAD) and 22 (56%) on oral anticoagulation (OAC). A pacemaker has previously been implanted in 1 (3%) patient, 19 (49%) patients suffered from hypertension, 15% from chronic obstructive pulmonary disease (COPD) and 7 (18%) patients had valvular heart disease (5 moderate mitral regurgitation, 1 moderate tricuspid regurgitation and 1 moderate aortic stenosis). A component of tachymyocardiopathy was documented in 8 (21%) patients.
Follow-up characteristics
During follow-up, 18 (46%) patients developed AF and in 12 of them AF was chronic. AFl recurrence was documented in 6 (15.4%) patients and all of them underwent a new successful ablation procedure. Mean (SD) time from ablation procedure to recurrence was 2.2 (1.8) years and mean (SD) time from first ablation procedure to redo was 2.7 (1.8) years. At the end of follow up 25 (64%) patients were in sinus rhythm, 12 (31%) in AF and 2 (5%) had pacemaker stimulation due to high-grade AV block.
A total of 7 patients had baseline valvular heart disease and 3 of them developed a high grade of valvular disease: severe mitral regurgitation (one), severe tricuspid regurgitation (one), and one severe aortic stenosis that underwent valvular replacement; while 4 patients with moderate mitral regurgitation developed mild regurgitation (functional mitral regurgitation due to reversible dilated cardiomiopathy).
Echocardiographic study
Table 2 summarizes echocardiographic parameters at baseline and at follow-up.
-

Table 2:
Differences between baseline and follow-up echocardiography study.
RAEDA: Right Atrium End Diastolic Area; RAESA: Right Atrium End Systolic Área; RACF: Right Atrium Contraction Fraction; LA: Left Atrium; DTE: Deceleration Time of E Wave; IVRT: Isovolumetric Time Relaxation Time; LVEF: Left Ventricular Eyection Fraction: LVEDD: Left Ventricular End Diastolic Diameter; LVESD: Left Ventricular End Systolic Diameter; IVS: Interventricular Septum; PW: Posterior Wall
Baseline study
Median left ventricular ejection fraction (LVEF) was 58%. In 82% of patients, diastolic dysfunction was observed and the most common diastolic dysfunction pattern was the type 3 (restrictive mitral filling pattern) (Figure 1). Median RACF was 0.30 and median RAEDA and RAESA were 23 cm2 and 17 cm2, respectively. LA median diameter was 44.8 mm and median area was 22.4 cm2. Median mitral A wave velocity was 0.5 m/s and mitral E wave velocity 0.9 m/s.
Follow-up study
Median LVEF was 62%. The most common mitral filling pattern was the type I (Figure 1). Median RACF was 0.40 and median RAEDA and RAESA were 21 cm2 and 13 cm2, respectively. LA median diameter was 46 mm and median area was 25 cm2. Median mitral A wave velocity was 0.76 m/s and mitral E wave velocity 0.78 m/s.
Echocardiographic atrial remodelling and diastolic function: differences between baseline and follow-up
There were differences between mitral A wave velocities and between E/A rates, with a significant increase in the mitral A wave velocity at follow-up with regard to baseline (Table 2). Significant differences were also found between RAEDA and RAESA, with lower dimensions at follow-up (Figure 2A), and there were also significant differences in the RACF with higher values at follow-up. No significant differences were detected in LA area, while LA diameter was significant larger at follow-up than baseline (Figure 2B).
-
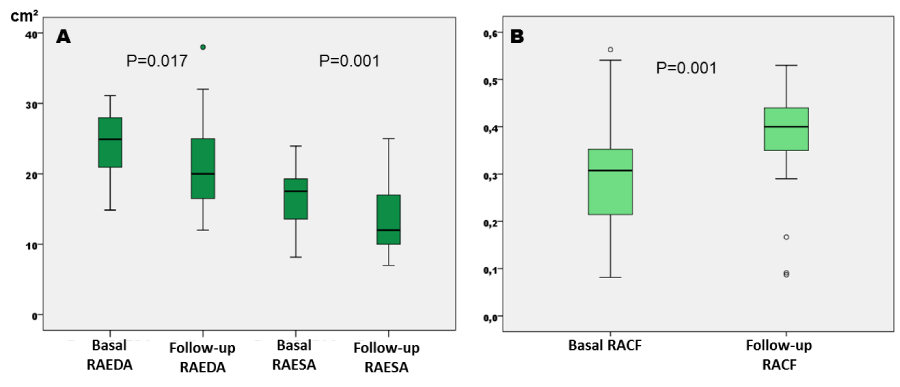
Figure 2
A: Differences between baseline and follow-up right atrial end-diastolic and end-systolic areas (cm²). B: Differences between right atrium contraction fraction at baseline and at follow-up. RAEDA: Right Atrium End-Diastolic Area. RAESA: Right Atrium End-Systolic Area. RACF: Right Atrium Contraction Fraction.
LA area and diastolic function in patients with AF during follow-up
Patients who developed AF trended to have a large LA than patients without AF, at baseline: 24 (20-25) cm2 vs. 21 (20-26) cm2, respectively, p= 0.403 and at follow-up: 26 (21-32) cm2 vs. 22 (20-27) cm2, respectively, p= 0.406.
Regarding to diastolic function, 15 of 18 patients (83%) who developed AF had baseline diastolic dysfunction and the most common type was type 3: 8 (44%) type 3, 4 (22%) type 2 and 3 (17%) type 1 (p= NS). Baseline diastolic dysfunction was also common in patients without AF during follow-up (79%), without differences between types of dysfunction.
Discussion
The main finding of our study was a RA reverse remodelling after typical AFl ablation. We observed a significant reduction in RAEDA and RAESA with an increase in the RACF. To the best of our knowledge, this is the first study that evaluates echocardiographic RA remodelling in a long-term follow-up (more over five years) in patients who underwent typical AFl ablation.
Several studies described structural reverse remodelling of the LA after pulmonary vein isolation as treatment of AF [3,4,11-15]. Muller et al. [16] documented a reverse remodelling in both atria after AF ablation.
In our study, we observed a reverse remodelling of the RA after typical AFl ablation, similar to the LA remodelling that has been previously described in patients who underwent pulmonary vein isolation. After typical AFl ablation the RA becomes smaller and improves its contractility suggesting a component of atrial tachymyocardiopathy after cardioversion of atrial tachyarrhythmia [17]. It is possible that these structural and functional changes after arrhythmia ablation and sinus rhythm restoration occur due to a component of tachymyocardiopathy, as occur in the left ventricle. It is well-established that a component of tachymyocardiopathy is reversible after an effective treatment [18-20], and we also observed this finding in our study, with a reduction of LVEDD and LVESD, and an increase in LVEF. The exact mechanisms that lead to the reversibility of myocardial dysfunction in patients with tachyarrhythmia are unknown. In experimental models [21,22] the dilated cardiomyopathy is induced with chronic high-frequency stimulation, and after cessation of the tachycardia, restoration of LVEF in the first weeks is observed, whereas diastolic volumes remain high for a period of up to three months or more and contractile dysfunction of myocytes may remain. These findings suggest the hypothesis of possible energy substrate repletion in myocytes as a mechanism of ventricular dysfunction. The cardiomyopathy induced by tachycardia is reversible in most cases. However, in patients with more left ventricular dilation and dysfunction and worse functional class, tachymyocardiopathy component may be irreversible. This could be explained by an alteration in the appearance of the architecture of myocardial fibrosis resulting in irreversible myocardial damage. It is probable that the same hypothesis of energy substrate repletion could be applied in the atrial myocardium, which would justify the reduction of RA dimensions and contractility recovery.
Moreover, the performance of one or more ablation lines at CTI may favour the development of a myocardial scar, which could facilitate RA retraction and hence reduction in its dimension.
Other studies [23,24] have demonstrated that patients with AFl shows advanced electrical RA remodelling and abnormal atrial substrate with reduction in regional voltage, an increase in low-voltage areas, slowed conduction and an increase in complex signals. It may be that in a long-term follow-up this electrical remodelling can manifest as structural reverse remodelling after ablation.
Another important finding was the slight enlargement of LA and the change in mitral pattern filling showing that diastolic function progressed from severe dysfunction at baseline to mild dysfunction in the long-term follow-up.
We observed LA dilation with respect to baseline and a significant increase in mitral A wave velocity. Possibly, the presence of left ventricular diastolic dysfunction can justify these findings, because of the relation between left ventricular diastolic pressure and LA pressure. At follow-up, may-be as a consequence of hypertension, more than half the patients had diastolic dysfunction, and the most common pattern was type I. Diastolic dysfunction causes an increase of left ventricle diastolic filling pressure, which leads to an increase in LA pressure that produces structural changes, atrial dilation and, initially, an increase of LA contractility to maintain left ventricular filling and cardiac output. Furthermore, diastolic dysfunction is related to the occurrence of AF [25]. We found 46% of patients had at least one episode of AF during follow-up, and in 31% AF become chronic. In our group of patients who developed AF, diastolic dysfunction was common (83%), and the most common pattern of dysfunction was type 3 (44%). In addition, these patients had larger LA than those without AF, at baseline and at follow-up. When LA enlargement occurs pulmonary veins also suffer distension and both changes favour the development of fibrosis and structural remodelling. This creates an ideal substrate that contributes to alter conduction properties, and makes the atrial myocardium more vulnerable to develop AF. On the other hand, it has also been documented that atrial enlargement may result from AF itself [26].
Limitations
This is a single centre study which included a small number of patients. More studies are necessary to assess RA remodelling after typical AFl ablation.
Structural remodelling was evaluated using 2D echocardiography. The availability of new imaging techniques, such as 3D echocardiography and cardiac magnetic resonance allow more accurate remodelling assessment and may be their measurements are less dependent of the operator. In addition, the echocardiography operators knew the purpose of this study. Diastolic function was evaluated using the mitral A wave velocity, the E wave velocity, the E/A ratio, the deceleration time of the E wave (DTE) and the isovolumetric relaxation time (IVRT) that are well established parameters to assess diastolic function and the E' was only used to distinguish filling pattern type II (E ‘/ A' <1, E ‘<8.5 cm/s) from normal filling pattern. The measurement of changes of E/E' is useful for diastolic function classification, and there are other parameters useful to assess diastolic function as the proBNP that were not included in this study.
Conclusion
AFl ablation favours a reverse remodelling of the right atrium and improves diastolic dysfunction parameters in the long-term follow-up.
- Vasan RS, Larson MG, Levy D, Galderisi M, Wolf PA, et al. (2003) Doppler transmitral flow indexes and risk of atrial fibrillation (the Framingham Heart Study). Am J Cardiol 91: 1079-1083.
- Thomas L, McKay T, Byth K, Marwick TH (2007) Abnormalities of left atrial function after cardioversion: An atrial strain rate study. Heart93: 89-95.
- Khan IA (2002) Transient atrial mechanical dysfunction (stunning) after cardioversion of atrial fibrillation and flutter. Am Heart Journal144: 11-22.
- Thomas L (2003) Atrial structural remodelling and restoration of atrial contraction after linear ablation for atrial fibrillation. Eur Heart J 24: 1942-1951.
- Kuppahally SS, Akoum N, Badger TJ, Burgon NS, Haslam T, et al. (2010) Echocardiographic left atrial reverse remodeling after catheter ablation of atrial fibrillation is predicted by preablation delayed enhancement of left atrium by magnetic resonance imaging. Am Heart J 160: 877-884.
- Waldo AL, Feld GK (2008) Inter-relationships of atrial fibrillation and atrial flutter mechanisms and clinical implications. J Am Coll Cardiol 51: 779-786.
- Sparks PB, Jayaprakash S, Vohra JK, Mond HG, Yapanis AG, et al. (1998) Left atrial "stunning" following radiofrequency catheter ablation of chronic atrial flutter. J Am Coll Cardiol 32: 468-475.
- Lang RM, Bierig M, Devereux RB, Flachskampf FA, Foster E, et al. (2005) Recommendations for chamber quantification: A report from the american society of echocardiography's guidelines and standards committee and the chamber quantification writing group, developed in conjunction with the european association of echocardiography, a branch of the european society of cardiology. J Am Soc Echocardiogr 18: 1440-1463.
- Lang RM, Bierig M, Devereux RB, Flachskampf FA, Foster E, et al. (2006) Recommendations for chamber quantification. Eur J Echocardiogr 7: 79-108.
- .
- Beukema WP, Elvan A, Sie HT, Misier AR, Wellens HJ (2005) Successful radiofrequency ablation in patients with previous atrial fibrillation results in a significant decrease in left atrial size. Circulation 112: 2089-2095.
- Reant P, Lafitte S, Jais P, Serri K, Weerasooriya, et al. (2005) Reverse remodeling of the left cardiac chambers after catheter ablation after 1 year in a series of patients with isolated atrial fibrillation. Circulation 112: 2896-2903.
- Jahnke C, Fischer J, Gerds-Li J-H, Gebker R, Manka R, et al. (2011) Serial monitoring of reverse left-atrial remodeling after pulmonary vein isolation in patients with atrial fibrillation: A magnetic resonance imaging study. Int J Cardiol153: 42-46.
- Montserrat S, Sitges M, Calvo N, Silva E, Tamborero D, et al. (2011) Effect of repeated radiofrequency catheter ablation on left atrial function for the treatment of atrial fibrillation. Am J Cardiol 108: 1741-1746.
- Richter B, Gwechenberger M, Socas A, Zorn G, Albinni S, et al. (2011) Time course of markers of tissue repair after ablation of atrial fibrillation and their relation to left atrial structural changes and clinical ablation outcome. Int J Cardiol 152: 231-236.
- Jayam VK, Dong J, Vasamreddy CR, Lickfett L, Kato R, et al. (2005) Atrial volume reduction following catheter ablation of atrial fibrillation and relation to reduction in pulmonary vein size: An evaluation using magnetic resonance angiography. J Interv Card Electrophysiol13: 107-114.
- Sanders P, Morton JB, Morgan JG, Davidson NC, Spence SJ, et al. (2002) Reversal of atrial mechanical stunning after cardioversion of atrial arrhythmias: Implications for the mechanisms of tachycardia-mediated atrial cardiomyopathy. Circulation106: 1806-1813 .
- Muller H, Noble S, Keller PF, Siqaud P, Gentil P, et al. (2008) Biatrial anatomical reverse remodelling after radiofrequency catheter ablation for atrial fibrillation: Evidence from real-time three-dimensional echocardiography. Europace 10: 1073-1078.
- Luchsinger JA, Steinberg JS (1998) Resolution of cardiomyopathy after ablation of atrial flutter. J Am Coll Cardiol 32: 205-210.
- Heinz G, Siostrzonek P, Kreiner G, Gössinger H (1992) Improvement in left ventricular systolic function after successful radiofrequency his bundle ablation for drug refractory, chronic atrial fibrillation and recurrent atrial flutter. Am J Cardiol 69: 489-492.
- Cruz FE, Cheriex EC, Smeets JL, Atié J, Peres AK, et al. (1990) Reversibility of tachycardia-induced cardiomyopathy after cure of incessant supraventricular tachycardia. J Am Coll Cardiol16: 739-744.
- Spinale FG (1991) Relation between ventricular and myocyte remodeling with the development and regression of supraventricular tachycardia-induced cardiomyopathy. Circ Res 69: 1058-1067.
- Stiles MK, Wong CX, John B, Kuklik P, Brooks AG, et al. (2010) Characterization of atrial remodeling studied remote from episodes of typical atrial flutter. Am J Cardiol 106: 528-534.
- Medi C, Teh AW, Roberts-Thomson K, Morton JB, Kistler PM, et al. (2012) Right atrial remodeling is more advanced in patients with atrial flutter than with atrial fibrillation. J Cardiovasc Electrophysiol 23: 1067-1072.
- Rosenberg MA, Gottdiener JS, Heckbert SR, Mukamal KJ (2012) Echocardiographic diastolic parameters and risk of atrial fibrillation: The cardiovascular health study. Eur Heart J 33: 904-912.
- Sanfilippo AJ, Abascal VM, Sheehan M, Oertel LB, Harrigan P, et al. (1990) Atrial enlargement as a consequence of atrial fibrillation. A prospective echocardiographic study. Circulation 82: 792-797.




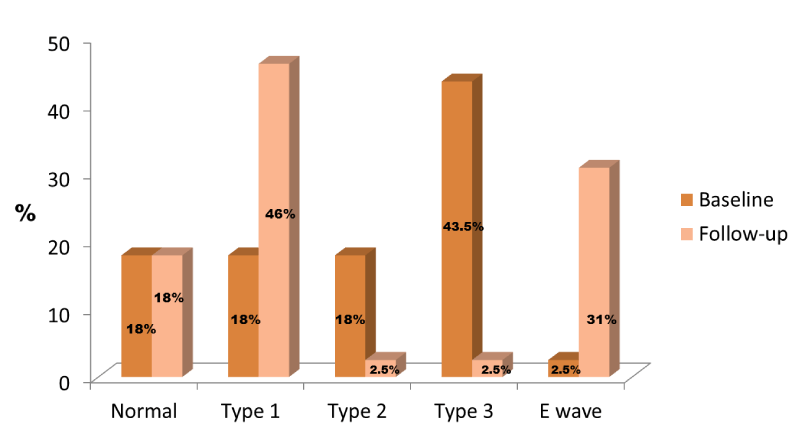





Table 1:
Baseline characteristics.
CABG: Coronary Arteries Bypass Graft; AFl: Atrial Flutter; AF: Atrial Fibrillation; COPD: Chronic Pulmonary Obstructive Disease
Table 1:
AFl type, n (%)
Paroxysmal
Persistent
16 (41)
Baseline characteristics.
CABG: Coronary Arteries Bypass Graft; AFl: Atrial Flutter; AF: Atrial Fibrillation; COPD: Chronic Pulmonary Obstructive Disease