Journal of Cardiovascular Medicine and Cardiology
Obesity: A Key Causative Factor in Cardiovascular Disease Development
Shuai Wang1, Ting He2, Chunli Mo1, Kangfeng Lin3,4, Cui Yang5 and Weihua Li1*
1Department of Cardiology, Xiamen Key Laboratory of Cardiac Electrophysiology, Xiamen Institute of Cardiovascular Diseases, The First Affiliated Hospital of Xiamen University, School of Medicine, Xiamen University, Xiamen 361003, China
2State Key Laboratory of Cellular Stress Biology, Innovation Center for Cell Signaling Network and Engineering, Research Center of Molecular Diagnostics of The Ministry of Education, School of Life Sciences, Xiamen University, Xiamen, Fujian, China
3School of Chemistry and Chemical Engineering, South China University of Technology, Guangzhou, China
4Yaneng Bioscience Co. Ltd., Shenzhen, China
5Department of Cardiology, Xiangyang No.1 People’s Hospital, Hubei University of Medicine, Xiangyang, Hubei, China
Cite this as
Wang S, He T, Mo C, Lin K, Yang C, Li W. Obesity: A Key Causative Factor in Cardiovascular Disease Development. J Cardiovasc Med Cardiol. 2024;11(4):090-095. Available from: 10.17352/2455-2976.000214Copyright License
© 2024 Wang S, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.Obesity and cardiovascular diseases have become one of the most pressing public health challenges of the 21st century, garnering widespread attention worldwide. Adipose depot expansion can occur through either an increase in the size of adipocytes (hypertrophy) or the generation of new adipocytes via precursor differentiation during adipogenesis (hyperplasia). Hypertrophy of adipocytes in obesity causes increased hypoxia, triggering fibrosis, inflammation, and necrosis, which contribute to systemic complications, including coronary artery disease, heart failure, and stroke. Furthermore, enlarged adipocytes release pro-inflammatory cytokines like TNF-α and IL-6, driving chronic inflammation that contributes to atherosclerosis, increasing the risk of heart attacks and strokes. We aim to discuss recent insights into the mechanisms by which obesity leads to cardiovascular diseases, which will provide new perspectives for the treatment of obesity and cardiovascular diseases.
Nonstandard abbreviations and acronyms
AGEs: Advanced Glycation End Products; AMP: AMP-Activated Protein Kinase; AMPK: AMP-Activated Protein Kinase; BMI: Body Mass Index; CVD: Cardiovascular Disease; FFAs: Free Fatty Acids; HDL: High-Density Lipoprotein; HFpEF: Heart Failure with Preserved Ejection Fraction; LDL: Low-Density Lipoprotein; LVH: Left Ventricular Hypertrophy; NAFLD: Non-Alcoholic Fatty Liver Disease; oxLDL: oxidized LDL; PPARα: Peroxisome Proliferator-Activated Receptor Alpha; RAAS: Renin-Angiotensin-Aldosterone System; TNF-α: Tumor Necrosis Factor-Alpha; VSMCs: Vascular Smooth Muscle Cells
Introduction
Obesity is a well-established risk factor for cardiovascular diseases (CVD), including atherosclerosis, heart failure, arrhythmias, thromboembolic events, and sudden cardiac death [1-4]. Despite significant advances in understanding the link between obesity and CVD [5], the exact mechanisms driving the onset and progression of these diseases remain incompletely understood. It is clear, however, that obesity induces a range of metabolic and physiological disruptions that contribute to cardiovascular pathogenesis [6]. Key mechanisms involved include insulin resistance, chronic inflammation, and dyslipidemia, all of which play pivotal roles in CVD development [7].
Furthermore, obesity and CVD form a vicious cycle: obesity leads to reduced physical activity, which in turn exacerbates metabolic disturbances, while cardiovascular diseases—through medication side effects, psychological stress, and persistent inflammation—can worsen obesity [8-10]. This bidirectional relationship highlights the need for a deeper understanding of the mechanisms linking obesity to cardiovascular and cerebrovascular diseases [11,12]. Such insights are crucial for developing effective treatment strategies that can break this cycle.
This review aims to provide an updated analysis of the pathophysiological mechanisms connecting obesity to cardiovascular and cerebrovascular diseases. By exploring these mechanisms, we hope to offer new perspectives for the prevention and treatment of obesity and its associated cardiovascular complications, ultimately advancing therapeutic approaches that target both conditions concurrently.
Metabolic dysregulation and its role in cardiovascular disease progression in obesity
Obesity is closely associated with a range of metabolic dysfunctions, including insulin resistance, dyslipidemia, and hypertension, which collectively exacerbate cardiovascular risk [13,14]. Insulin resistance, a central feature of obesity, significantly disrupts endothelial function, which is crucial for vascular health [15]. In this condition, reduced cellular sensitivity to insulin impairs glucose uptake, leading to hyperglycemia [16]. Elevated glucose levels promote the formation of Advanced Glycation End products (AGEs), which accumulate in the vasculature and induce oxidative stress [17,18]. This oxidative damage impairs endothelial nitric oxide (NO) production, essential for vascular tone and integrity, resulting in endothelial dysfunction [19,20]. The reduced availability of NO contributes to endothelial cell injury, a pro-thrombotic state, and the dysfunction of vascular smooth muscle cells, ultimately promoting the development of atherosclerosis [21-23]. This cascade of events accelerates arterial plaque formation, leading to vascular narrowing and stiffening, which increases the risk of cardiovascular diseases [24,25].
Inflammatory responses and adipokines
Obesity is characterized by altered adipokine secretion, where adipocytes release a variety of bioactive molecules that play crucial roles in regulating metabolic and inflammatory processes [26,27]. In lean individuals, anti-inflammatory cytokines primarily activate M2 macrophages and inhibit neutrophil-mediated inflammation, thereby promoting an overall anti-inflammatory environment [28]. Adipocytes secrete adiponectin and a range of anti-inflammatory cytokines, including Interleukin-10 (IL-10), Interleukin-4 (IL-4), and Interleukin-13 (IL-13) [27]. Adiponectin enhances endothelial antioxidant capacity, inhibits vascular smooth muscle cell proliferation, and promotes fatty acid oxidation by activating AMP-Activated Protein Kinase (AMPK) and peroxisome Proliferator-Activated Receptor Alpha (PPAR-α), thereby reducing the formation of atherosclerotic plaques [29]. IL-10, IL-4, and IL-13 help alleviate chronic low-grade inflammation by inhibiting macrophage polarization to the pro-inflammatory M1 phenotype and reducing the secretion of other inflammatory mediators, ultimately preserving vascular homeostasis [30]. However, in individuals with obesity, adipocytes secrete excessive amounts of pro-inflammatory cytokines, such as Tumor Necrosis Factor-Alpha (TNF-α), Interleukin-6 (IL-6), resistin, and leptin, which subsequently activate a pro-inflammatory M1 macrophage response [31,32]. TNF-α and IL-6 activate key intracellular signaling pathways, including the NF-κB and JAK/STAT pathways, which upregulate the expression of adhesion molecules (such as ICAM-1 and VCAM-1) on endothelial cells, facilitating the recruitment of inflammatory cells to the vascular walls [33]. Additionally, leptin and resistin contribute by enhancing oxidative stress and further stimulating pro-inflammatory cytokine production, such as IL-1β and TNF-α, in macrophages [34,35]. This creates a positive feedback loop that sustains and amplifies the inflammatory response. As a result, the increased secretion of inflammatory mediators accelerates endothelial dysfunction, promotes lipid accumulation, and fosters the development of atherosclerotic plaques. These adipokines contribute to a state of chronic low-grade inflammation, which has been identified as a key driver of endothelial dysfunction [36]. Chronic inflammation promotes the recruitment of immune cells to sites of vascular injury, further amplifying the inflammatory cascade and facilitating the development of atherosclerosis [37,38] (Figure 1).
In addition to their role in inflammation, elevated adipokine levels disrupt the balance between vasodilators and vasoconstrictors [39]. This imbalance exacerbates vascular dysfunction by impairing the normal regulation of vascular tone. The resultant dysregulation of vascular tone contributes to the development of hypertension and accelerates the progression of atherosclerotic lesions [40,41]. Collectively, these inflammatory and adipokine-mediated alterations in vascular function represent critical mechanisms by which obesity promotes cardiovascular pathology.
In lean individuals, anti-inflammatory cytokines activate M2 macrophages and suppress neutrophil-driven inflammation. In obese individuals, hypertrophic or apoptotic adipocytes in obesity secrete pro-inflammatory molecules, such as leptin, resistin, IL-6, and TNF-α, which trigger an M1 macrophage response.
Altered lipid metabolism and atherosclerosis
Obesity induces significant disruptions in lipid metabolism, primarily through the accumulation of visceral fat, which elevates circulating Free Fatty Acids (FFAs) [42,43]. These FFAs are taken up by various tissues, including the liver, heart, and vasculature, contributing to Non-Alcoholic Fatty Liver Disease (NAFLD) and dyslipidemia, characterized by increased low-density lipoprotein (LDL) cholesterol and decreased High-Density Lipoprotein (HDL) cholesterol [44,45]. The elevated LDL infiltrates the subendothelial space, where it undergoes oxidation to form oxidized LDL (oxLDL). OxLDL is a potent inflammatory mediator that promotes the recruitment of circulating monocytes, which then differentiate into macrophages within the subendothelial space [46]. These macrophages internalize oxLDL, transforming into foam cells and contributing to plaque formation. The accumulation of foam cells triggers a sustained inflammatory response, which attracts additional immune cells, establishing a self-perpetuating cycle of inflammation and lipid deposition [47]. Concurrently, vascular smooth muscle cells (VSMCs) migrate to the site of the plaque, proliferate, and may transdifferentiate into foam cells, further exacerbating plaque expansion [48]. This process not only accelerates the progression of atherosclerotic lesions but also enhances plaque instability, setting the stage for potential rupture and subsequent thrombotic events [49,50]. Simultaneously, increased FFAs impair endothelial function and exacerbate lipid accumulation, further advancing plaque progression. Fat accumulation in the vascular wall increases oxidative stress, destabilizing plaques and heightening the risk of rupture [51].
Plaque rupture exposes thrombogenic material to the bloodstream, triggering platelet aggregation and thrombus formation, which significantly raises the risk of myocardial infarction and stroke. Therefore, obesity-induced alterations in lipid metabolism—through effects on LDL, FFAs, and oxidative stress—play a central role in atherosclerosis and its cardiovascular complications [52].
Mechanical stress and cardiac remodeling
Obesity imposes mechanical stress on the heart due to increased adipose tissue volume, which demands higher blood flow and elevates cardiac output [53]. Over time, this results in Left Ventricular Hypertrophy (LVH), where the left ventricle enlarges and thickens in response to the added workload. LVH can progress to Heart Failure with Preserved Ejection Fraction (HFpEF), a condition frequently observed in obese individuals [54]. Additionally, increased abdominal visceral fat contributes to systemic hypertension by compressing the kidneys and activating the Renin-Angiotensin-Aldosterone System (RAAS), a key blood pressure regulator [55]. This hypertension further raises the risk of cerebrovascular events, such as stroke [56]. Thus, the mechanical overload and associated hemodynamic changes from obesity contribute to both heart failure and stroke, creating a vicious cycle that exacerbates cardiovascular morbidity.
Discussion
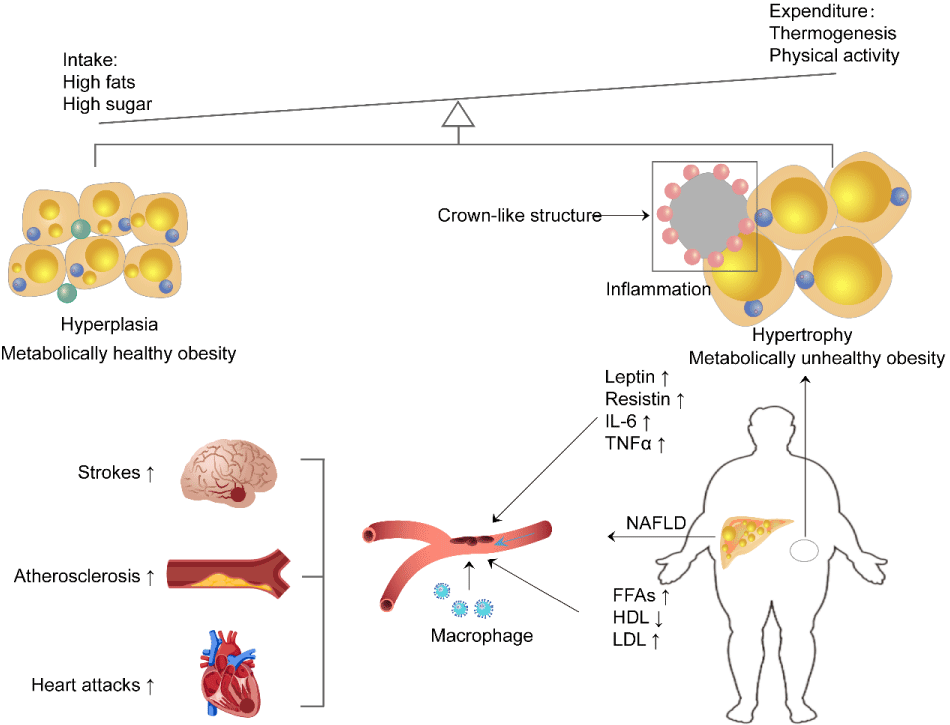
In summary, obesity (especially hypertrophic obesity) significantly contributes to the development and progression of cardiovascular and cerebrovascular diseases through a series of interconnected mechanisms. Metabolic dysregulation, including insulin resistance, dyslipidemia, and hypertension, plays a central role in promoting endothelial dysfunction and accelerating atherosclerosis. Chronic inflammation driven by altered adipokine secretion exacerbates vascular damage, while increased oxidative stress and lipid accumulation further contribute to the formation of atherosclerotic plaques. Additionally, mechanical stress induced by excess adipose tissue results in cardiac remodeling, increasing the risk of heart failure and stroke. These interconnected pathways underscore the critical role of obesity in cardiovascular pathology (Figure 2).
When energy intake far exceeds expenditure, the excess energy is stored in fat, leading to either adipocyte hyperplasia (healthy obesity) or hypertrophy (unhealthy obesity). Hypertrophic adipocytes secrete adipokines, including TNF-α and IL-6, which induce chronic inflammation. Meanwhile, the decrease in HDL and the increase in LDL and free fatty acids contribute to the development of fatty liver. Together, these factors promote atherosclerosis, stroke, heart disease, and other related conditions.
Obesity is a well-established risk factor for CVD. However, emerging research has introduced the “obesity paradox,” which suggests that individuals with obesity and pre-existing CVD may have better survival outcomes compared to their lean counterparts [57]. This paradox may be explained by factors such as higher muscle mass, better physical fitness, and the potential benefits of interventions like weight loss or increased physical activity. These findings imply that while obesity remains a significant risk factor for CVD in the general population, its impact may be less detrimental in individuals with established CVD, depending on factors like fitness, muscle mass, and metabolic health. Nevertheless, the obesity paradox does not negate the overwhelming evidence linking obesity to increased CVD risk in the broader population [32]. Further research is needed to elucidate the mechanisms underlying this paradox and its clinical implications. Future studies should explore how interventions can mitigate the effects of obesity on cardiovascular health and whether personalized treatment strategies, considering metabolic and genetic profiles, can optimize outcomes.
Research on obesity-induced CVD faces several key challenges. The complex interplay between genetic, environmental, and lifestyle factors remains insufficiently understood, and the precise molecular mechanisms linking inflammation and lipid metabolism to CVD are yet to be fully elucidated. Moreover, the heterogeneous nature of obesity—shaped by individual metabolic profiles, genetic predispositions, and comorbidities—complicates the development of universal therapeutic strategies. Current animal models and clinical studies often fail to accurately replicate human conditions or account for the multifactorial aspects of obesity. Future research should prioritize personalized approaches that integrate genetic and metabolic factors, enabling a deeper understanding of individual responses to obesity-related CVD. This could help identify more effective, targeted interventions for prevention and treatment, advancing the field toward precision medicine in cardiovascular care. Looking ahead, a more comprehensive understanding of the mechanisms underlying obesity-induced CVD could facilitate the development of more targeted prevention and treatment strategies. The first priority should be weight management, including reducing the intake of high-calorie foods and increasing physical activity. Furthermore, critical pathways warranting attention include inflammation, lipid metabolism, and insulin resistance, with particular emphasis on the roles of pro-inflammatory cytokines, oxidative stress, and adipokines in driving endothelial dysfunction and atherosclerosis. Targeting specific molecular mediators such as TNF-α, IL-6, and NF-κB may help mitigate the inflammatory burden associated with obesity. In addition, therapies aimed at improving lipid metabolism, particularly those targeting dysregulated HDL and LDL cholesterol, hold considerable promise. Personalized cardiovascular care, tailored to individual dietary habits, metabolic profiles, and genetic factors, is vital for optimizing treatment and enabling more precise interventions. As the global prevalence of obesity continues to rise, addressing these underlying mechanisms will be crucial in alleviating the long-term burden of CVD and improving patient outcomes.
Author contributions
S. W. had the idea for this article and drafted the manuscript. T.H., C.-L.M., K.-F.L. and C.Y. performed the literature search. W.-H.L. supervised the project, revised the manuscript and provided fundings.
Funding
This work was supported by grants from Science and Technology Planning Projects of Xiamen (3502Z20224021 to W.L.), Postdoctoral Fellowship Program of CPSF under Grant Number GZC20231412 to T.H.
- Commodore-Mensah Y, Lazo M, Tang O, Echouffo-Tcheugui JB, Ndumele CE, Nambi V, et al. High burden of subclinical and cardiovascular disease risk in adults with metabolically healthy obesity: the Atherosclerosis Risk in Communities (ARIC) Study. Diabetes Care. 2021;44(7):1657-1663. Available from: https://doi.org/10.2337/dc20-2227
- Kachur S, Lavie CJ, de Schutter A, Milani RV, Ventura HO. Obesity and cardiovascular diseases. Minerva Med. 2017;108(3):212-228. Available from: https://doi.org/10.23736/s0026-4806.17.05022-4
- Marfella R, D'Amico M, Di Filippo C, Siniscalchi M, Sasso FC, Ferraraccio F, et al. The possible role of the ubiquitin proteasome system in the development of atherosclerosis in diabetes. Cardiovasc Diabetol. 2007;6:35. Available from: https://doi.org/10.1186/1475-2840-6-35
- Tromp J, Paniagua SMA, Lau ES, Allen NB, Blaha MJ, Gansevoort RT, et al. Age dependent associations of risk factors with heart failure: pooled population based cohort study. BMJ. 2021;372:n461. Available from: https://doi.org/10.1136/bmj.n461
- Nyawo TA, Pheiffer C, Mazibuko-Mbeje SE, Mthembu SXH, Nyambuya TM, Nkambule BB, et al. Physical exercise potentially targets epicardial adipose tissue to reduce cardiovascular disease risk in patients with metabolic diseases: oxidative stress and inflammation emerge as major therapeutic targets. Antioxidants (Basel). 2021;10(11):1758. Available from: https://doi.org/10.3390/antiox10111758
- Tesauro M, Cardillo C. Obesity, blood vessels and metabolic syndrome. Acta Physiol (Oxf). 2011;203(3):279-286. Available from: https://doi.org/10.1111/j.1748-1716.2011.02290.x
- Francisco V, Ruiz-Fernández C, Pino J, Mera A, González-Gay MA, Gómez R, et al. Adipokines: linking metabolic syndrome, the immune system, and arthritic diseases. Biochem Pharmacol. 2019;165:196-206. Available from: https://doi.org/10.1016/j.bcp.2019.03.030
- Pannu S, Rosmarin D. Psoriasis in patients with metabolic syndrome or type 2 diabetes mellitus: treatment challenges. Am J Clin Dermatol. 2021;22(3):293-300. Available from: https://doi.org/10.1007/s40257-021-00590-y
- Lambert EA, Straznicky NE, Dixon JB, Lambert GW. Should the sympathetic nervous system be a target to improve cardiometabolic risk in obesity? Am J Physiol Heart Circ Physiol. 2015;309(3):H244-258. Available from: https://doi.org/10.1152/ajpheart.00096.2015
- Otani H. Oxidative stress as pathogenesis of cardiovascular risk associated with metabolic syndrome. Antioxid Redox Signal. 2011;15(7):1911-1926. Available from: https://doi.org/10.1089/ars.2010.3739
- Evans LE, Taylor JL, Smith CJ, Pritchard HAT, Greenstein AS, Allan SM. Cardiovascular comorbidities, inflammation, and cerebral small vessel disease. Cardiovasc Res. 2021;117(13):2575-2588. Available from: https://doi.org/10.1093/cvr/cvab284
- Ząbczyk M, Ariëns RAS, Undas A. Fibrin clot properties in cardiovascular disease: from basic mechanisms to clinical practice. Cardiovasc Res. 2023;119(1):94-111. Available from: https://doi.org/10.1093/cvr/cvad017
- Bishop NC, Wadley AJ, Hamrouni M, Roberts MJ. Inactivity and obesity: consequences for macrophage-mediated inflammation and the development of cardiometabolic disease. Proceedings of the Nutrition Society. 2023;82:13-21. Available from: https://doi.org/10.1017/s0029665122002671
- Wärnberg J, Cunningham K, Romeo J, Marcos A. Physical activity, exercise and low-grade systemic inflammation. Proceedings of the Nutrition Society. 2010;69(4):400-406. Available from: https://doi.org/10.1017/s0029665110001928
- Bigazzi R, Bianchi S. Insulin resistance, metabolic syndrome and endothelial dysfunction. J Nephrol. 2007;20(1):10-14. Available from: https://pubmed.ncbi.nlm.nih.gov/17347967/
- Yang J, Zhang LJ, Wang F, Hong T, Liu Z. Molecular imaging of diabetes and diabetic complications: beyond pancreatic β-cell targeting. Adv Drug Deliv Rev. 2019;139:32-50. Available from: https://doi.org/10.1016/j.addr.2018.11.007
- Rader DJ. Effect of insulin resistance, dyslipidemia, and intra-abdominal adiposity on the development of cardiovascular disease and diabetes mellitus. Am J Med. 2007;120(1 Suppl):S12-18. Available from: https://doi.org/10.1016/j.amjmed.2007.01.003
- Liu J, Shen W, Zhao B, Wang Y, Wertz K, Weber P, et al. Targeting mitochondrial biogenesis for preventing and treating insulin resistance in diabetes and obesity: hope from natural mitochondrial nutrients. Adv Drug Deliv Rev. 2009;61(13):1343-1352. Available from: https://doi.org/10.1016/j.addr.2009.06.007
- Li R, Lau WB, Ma XL. Adiponectin resistance and vascular dysfunction in the hyperlipidemic state. Acta Pharmacol Sin. 2010;31(10):1258-1266. Available from: https://doi.org/10.1038/aps.2010.95
- Higashi Y, Sasaki S, Nakagawa K, Matsuura H, Chayama K, Oshima T. Effect of obesity on endothelium-dependent, nitric oxide-mediated vasodilation in normotensive individuals and patients with essential hypertension. Am J Hypertens. 2001;14(10):1038-1045. Available from: https://doi.org/10.1016/s0895-7061(01)02191-4
- Wang S, Wang Y, Lai X, Sun J, Hu M, Chen M, et al. Minimalist nanocomplex with dual regulation of endothelial function and inflammation for targeted therapy of inflammatory vascular diseases. ACS Nano. 2023;17(3):2761-2781. Available from: https://doi.org/10.1021/acsnano.2c11058
- Li J, Wang W, Han L, Feng M, Lu H, Yang L, et al. Human apolipoprotein A-I exerts a prophylactic effect on high-fat diet-induced atherosclerosis via inflammation inhibition in a rabbit model. Acta Biochim Biophys Sin (Shanghai). 2017;49(2):149-158. Available from: https://doi.org/10.1093/abbs/gmw128
- Guzik TJ, Skiba DS, Touyz RM, Harrison DG. The role of infiltrating immune cells in dysfunctional adipose tissue. Cardiovasc Res. 2017;113(9):1009-1023. Available from: https://doi.org/10.1093/cvr/cvx108
- Jiang Q, Liu H, Wang S, Wang J, Tang Y, He Z, et al. Circadian locomotor output cycles kaput accelerates atherosclerotic plaque formation by upregulating plasminogen activator inhibitor-1 expression. Acta Biochim Biophys Sin (Shanghai). 2018;50(9):869-879. Available from: https://doi.org/10.1093/abbs/gmy087
- Ren K, Xu XD, Yu XH, Li MQ, Shi MW, Liu QX, et al. LncRNA-modulated autophagy in plaque cells: a new paradigm of gene regulation in atherosclerosis? Aging (Albany NY). 2020;12(21):22335-22349. Available from: https://doi.org/10.18632/aging.103786
- Wrońska A, Kmiec Z. Structural and biochemical characteristics of various white adipose tissue depots. Acta Physiol (Oxf). 2012;205(2):194-208. Available from: https://doi.org/10.1111/j.1748-1716.2012.02409.x
- Sirota P, Hadi E, Djaldetti M, Bessler H. Difference in inflammatory cytokine production by mononuclear cells from obese and non-obese schizophrenic patients. Acta Psychiatr Scand. 2015;132(4):301-305. Available from: https://doi.org/10.1111/acps.12396
- Ho PY, Chou YC, Koh YC, Lin WS, Chen WJ, Tseng AL, et al. Lactobacillus rhamnosus 069 and Lactobacillus brevis 031: unraveling strain-specific pathways for modulating lipid metabolism and attenuating high-fat-diet-induced obesity in mice. ACS Omega. 2024;9(26):28520-28533. Available from: https://doi.org/10.1021/acsomega.4c02514
- Mahadik SR, Lele RD, Saranath D, Seth A, Parikh V. Uncoupling protein-2 (UCP2) gene expression in subcutaneous and omental adipose tissue of Asian Indians: relationship to adiponectin and parameters of metabolic syndrome. Adipocyte. 2012;1(2):101-107. Available from: https://doi.org/10.4161/adip.19671
- Biswas SK, Chittezhath M, Shalova IN, Lim JY. Macrophage polarization and plasticity in health and disease. Immunol Res. 2012;53(1-3):11-24. Available from: https://doi.org/10.1007/s12026-012-8291-9
- Vettor R, Milan G, Rossato M, Federspil G. Review article: adipocytokines and insulin resistance. Aliment Pharmacol Ther. 2005;22 Suppl 2:3-10. Available from: https://doi.org/10.1111/j.1365-2036.2005.02587.x
- Vecchié A, Dallegri F, Carbone F, Bonaventura A, Liberale L, Portincasa P, et al. Obesity phenotypes and their paradoxical association with cardiovascular diseases. Eur J Intern Med. 2018;48:6-17. Available from: https://doi.org/10.1016/j.ejim.2017.10.020
- Bhol NK, Bhanjadeo MM, Singh AK, Dash UC, Ojha RR, Majhi S, et al. The interplay between cytokines, inflammation, and antioxidants: mechanistic insights and therapeutic potentials of various antioxidants and anti-cytokine compounds. Biomed Pharmacother. 2024;178:117177. Available from: https://doi.org/10.1016/j.biopha.2024.117177
- Webster JD, Vucic D. The balance of TNF-mediated pathways regulates inflammatory cell death signaling in healthy and diseased tissues. Front Cell Dev Biol. 2020;8:365. Available from: https://doi.org/10.3389/fcell.2020.00365
- Brasier AR. The nuclear factor-kappaB-interleukin-6 signaling pathway mediating vascular inflammation. Cardiovasc Res. 2010;86(2):211-218. Available from: https://doi.org/10.1093/cvr/cvq076
- Feijóo-Bandín S, Aragón-Herrera A, Moraña-Fernández S, Anido-Varela L, Tarazón E, Roselló-Lletí E, Portolés M, et al. Adipokines and inflammation: Focus on cardiovascular diseases. Int J Mol Sci. 2020;21(20):7711. Available from: https://doi.org/10.3390/ijms21207711
- Livshits G, Kalinkovich A. Inflammaging as a common ground for the development and maintenance of sarcopenia, obesity, cardiomyopathy and dysbiosis. Ageing Res Rev. 2019;56:100980. Available from: https://doi.org/10.1016/j.arr.2019.100980
- Tian XY, Ganeshan K, Hong C, Nguyen KD, Qiu Y, Kim J, et al. Thermoneutral housing accelerates metabolic inflammation to potentiate atherosclerosis but not insulin resistance. Cell Metab. 2016;23(1):165-178. Available from: https://doi.org/10.1016/j.cmet.2015.10.003
- Saxton SN, Ryding KE, Aldous RG, Withers SB, Ohanian J, Heagerty AM. Role of sympathetic nerves and adipocyte catecholamine uptake in the vasorelaxant function of perivascular adipose tissue. Arterioscler Thromb Vasc Biol. 2018;38(4):880-891. Available from: https://doi.org/10.1161/atvbaha.118.310777
- Verma S, Li SH, Wang CH, Fedak PW, Li RK, Weisel RD, et al. Resistin promotes endothelial cell activation: further evidence of adipokine-endothelial interaction. Circulation. 2003;108(6):736-740. Available from: https://doi.org/10.1161/01.cir.0000084503.91330.49
- Schinzari F, Tesauro M, Rovella V, Di Daniele N, Mores N, Veneziani A, et al. Leptin stimulates both endothelin-1 and nitric oxide activity in lean subjects but not in patients with obesity-related metabolic syndrome. J Clin Endocrinol Metab. 2013;98(3):1235-1241. Available from: https://doi.org/10.1210/jc.2012-3424
- Nosrati-Oskouie M, Aghili-Moghaddam NS, Sathyapalan T, Sahebkar A. Impact of curcumin on fatty acid metabolism. Phytother Res. 2021;35(9):4748-4762. Available from: https://doi.org/10.1002/ptr.7105
- Lee-Rueckert M, Canyelles M, Tondo M, Rotllan N, Kovanen PT, Llorente-Cortes V, et al. Obesity-induced changes in cancer cells and their microenvironment: mechanisms and therapeutic perspectives to manage dysregulated lipid metabolism. Semin Cancer Biol. 2023;93:36-51. Available from: https://doi.org/10.1016/j.semcancer.2023.05.002
- Perna S, Rafique A, Rondanelli M, Allehdan S, Riso P, Marino M. Effect of caper fruit (Capparis spinosa L.) consumption on liver enzymes, lipid profile, fasting plasma glucose, and weight loss. A systematic review and a preliminary meta-analysis of randomized controlled trials. Biomed Pharmacother. 2023;168:115638. Available from: https://doi.org/10.1016/j.biopha.2023.115638
- Rezaei S, Tabrizi R, Nowrouzi-Sohrabi P, Jalali M, Shabani-Borujeni M, Modaresi S, et al. The effects of vitamin D supplementation on anthropometric and biochemical indices in patients with non-alcoholic fatty liver disease: a systematic review and meta-analysis. Front Pharmacol. 2021;12:732496. Available from: https://doi.org/10.3389/fphar.2021.732496
- Libby P, Ridker PM, Maseri A. Inflammation and atherosclerosis. Circulation. 2002;105(11):1135-1143. Available from: https://doi.org/10.1161/hc0902.104353
- Bennett MR, Sinha S, Owens GK. Vascular smooth muscle cells in atherosclerosis. Circ Res. 2016;118(4):692-702. Available from: https://doi.org/10.1161/circresaha.115.306361
- Libby P. The changing landscape of atherosclerosis. Nature. 2021;592(7852):524-533. Available from: https://doi.org/10.1038/s41586-021-03392-8
- Liu L, Lan X, Chen X, Dai S, Wang Z, Zhao A, et al. Multi-functional plant flavonoids regulate pathological microenvironments for vascular stent surface engineering. Acta Biomater. 2023;157:655-669. Available from: https://doi.org/10.1016/j.actbio.2022.11.035
- Dussault S, Dhahri W, Desjarlais M, Mathieu R, Rivard A. Elsibucol inhibits atherosclerosis following arterial injury: multifunctional effects on cholesterol levels, oxidative stress and inflammation. Atherosclerosis. 2014;237(1):194-199. Available from: https://doi.org/10.1016/j.atherosclerosis.2014.09.008
- Liu H, Yin G, Kohlhepp MS, Schumacher F, Hundertmark J, Hassan MIA, Heymann F, et al. Dissecting acute drug-induced hepatotoxicity and therapeutic responses of steatotic liver disease using primary mouse liver and blood cells in a liver-on-a-chip model. Adv Sci (Weinh). 2024;11(30):e2403516. Available from: https://doi.org/10.1002/advs.202403516
- Ridker PM. High-sensitivity C-reactive protein: potential adjunct for global risk assessment in the primary prevention of cardiovascular disease. Circulation. 2001;103(13):1813-1818. Available from: https://doi.org/10.1161/01.cir.103.13.1813
- Messerli FH, Sundgaard-Riise K, Reisin ED, Dreslinski GR, Ventura HO, Oigman W, et al. Dimorphic cardiac adaptation to obesity and arterial hypertension. Ann Intern Med. 1983;99(6):757-761. Available from: https://doi.org/10.7326/0003-4819-99-6-757
- Hicklin HE, Gilbert ON, Ye F, Brooks JE, Upadhya B. Hypertension as a road to treatment of heart failure with preserved ejection fraction. Curr Hypertens Rep. 2020;22(8):82. Available from: https://doi.org/10.1007/s11906-020-01093-7
- Rao A, Pandya V, Whaley-Connell A. Obesity and insulin resistance in resistant hypertension: implications for the kidney. Adv Chronic Kidney Dis. 2015;22(3):211-217. Available from: https://doi.org/10.1053/j.ackd.2014.12.004
- Jamerson KA. Rationale for angiotensin II receptor blockers in patients with low-renin hypertension. Am J Kidney Dis. 2000;36(1 Suppl 1):S24-30. Available from: https://doi.org/10.1053/ajkd.2000.9688
- Lavie CJ, Milani RV, Ventura HO. Obesity and cardiovascular disease: risk factor, paradox, and impact of weight loss. J Am Coll Cardiol. 2009;53(21):1925-1932. Available from: https://doi.org/10.1016/j.jacc.2008.12.068