Journal of Cardiovascular Medicine and Cardiology
Refractory Ventricular Tachycardia Storm: A case report and review of literature
Navjyot Kaur*
Cite this as
Kaur N (2019) Refractory Ventricular Tachycardia Storm: A case report and review of literature. J Cardiovasc Med Cardiol 6(3): 045-050. DOI: 10.17352/2455-2976.000091Ventricular tachycardia (VT) storm continues to be an important challenge for the cardiologists all over the globe. VT storm usually occurs in a structurally diseased heart with low left ventricular ejection fraction (LVEF) but may also occur in patients with arrhythmic syndromes like long QT syndrome, Brugada syndrome and catecholaminergic polymorphic VT who have structurally normal heart. Despite its life threatening consequences, the guidelines and literature regarding management of incessant VT is sparse. Here we present a middle age male who presented with scar related refractory VT. Unfortunately we lost the patient; but this case highlights the difficulty and challenges faced in management of hemodynamically unstable VT storm.
Case Report
58 years old male, a chronic smoker with smoking index > 800 and a heavy alcohol consumer (140-160 gm/day since more than 20 years) and a known case of chronic obstructive pulmonary disease (COPD) was brought to emergency of a cardiac center in Punjab on 4th April 2019 in cardiogenic shock (CS) and pulmonary edema. There was history of chest pain since last 15 days. Evaluation revealed anterior wall myocardial infarction (AWMI), severe left ventricular dysfunction (left ventricular ejection fraction [LVEF] : 25%) with left anterior descending (LAD) artery territory akinetic, N-terminal pro b-type natriuretic peptide (NT-pro BNP) of 4100 pg/ml and chest x ray evidence of pulmonary edema. The blood gas analysis revealed severe metabolic acidosis with hypoxia. The patient suffered cardiac arrest and was found to have polymorphic ventricular tachycardia (VT). He was resuscitated with direct cardioversion (DC) followed by cardio pulmonary resuscitation (CPR) and was taken up for revascularization. RESOLUTE ONYX (MEDTRONIC) 3.5 mm X 26 mm stent was placed in proximal LAD. Serum electrolytes including potassium, magnesium and calcium were maintained within normal limit. Thyroid profile was normal. During admission he was documented to have recurrent monomorphic VT. The QTc was prolonged hence he was managed with xylocard bolus & infusion followed by oral mexilitene and betablockers. He was discharged from above center on 15 April 19 in hemodynamically stable condition and ECG on discharge revealed heart rate of 63/minute, T wave inversion in V1-V6 & I, aVL leads & occasional ventricular premature complexes (VPCs).
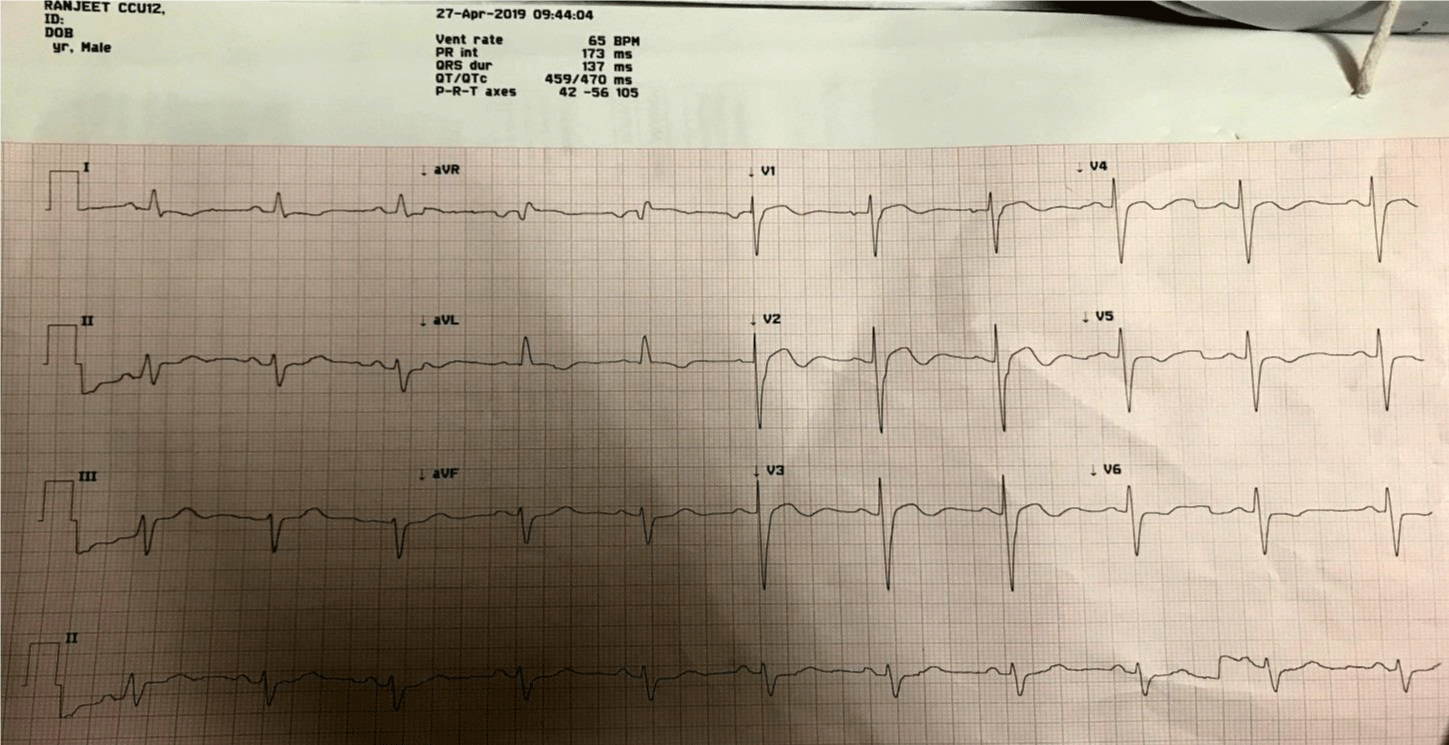
On 23 April 2019, patient was admitted at advance cardiac center, Post Graduate Institute of Medical Education & Research (PGIMER), Chandigarh with complains of shortness of breath (NYHA II-III) & episodes of sudden onset palpitations associated with presycope. On admission; he had heart rate of 150/min with blood pressure of 96/70 mm of Hg. Chest revealed bilateral basal crepts. ECG revealed monomorphic VT. The patient was given a bolus of xylocard (1.5mg/kg); the rhythm reverted to sinus with frequent VPCs. He was started on xylocard infusion (1mg/kg/hr) while oral mexilitene (200 mg TDS) was continued and long acting propranolol (40 mg TDS) was added. Potassium was 4.4 meq/L while magnesium was 2.5 mg/dL. 2D Echo revealed severe LV dysfunction (EF-20-25%) with akinetic LAD territory. During first 24 hours; there were frequent runs of NSVT; hence right stellate ganglion blockade (SGB) was done with bupivacaine. Post procedure; the VPC load reduced significantly for 24 hours however there was recurrence of NSVT on 26th April; hence the right SGB was done second time. For around 36 hours patient remained asymptomatic. His heart rate was between 56-66/min with normal QTc and very occasional VPCs (Figure 1). Rest Myocardial Perfusion Scintigraphy revealed LVEF of 30% at rest with 50% myocardium being non-viable mainly corresponding to LAD territory.
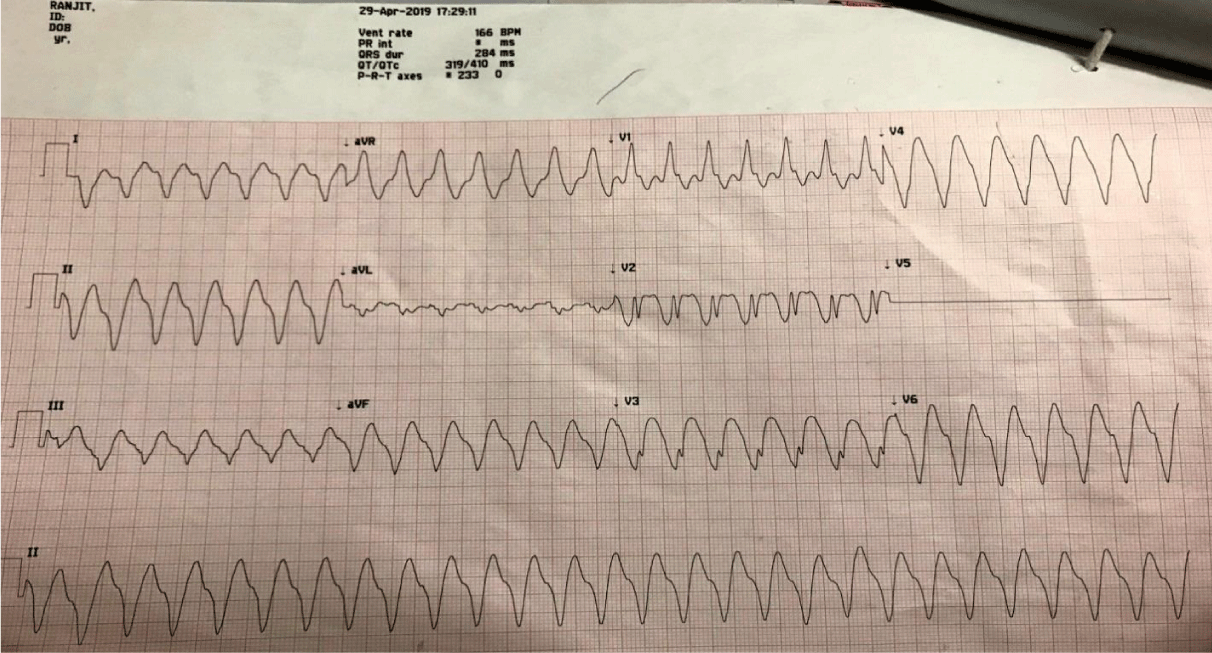
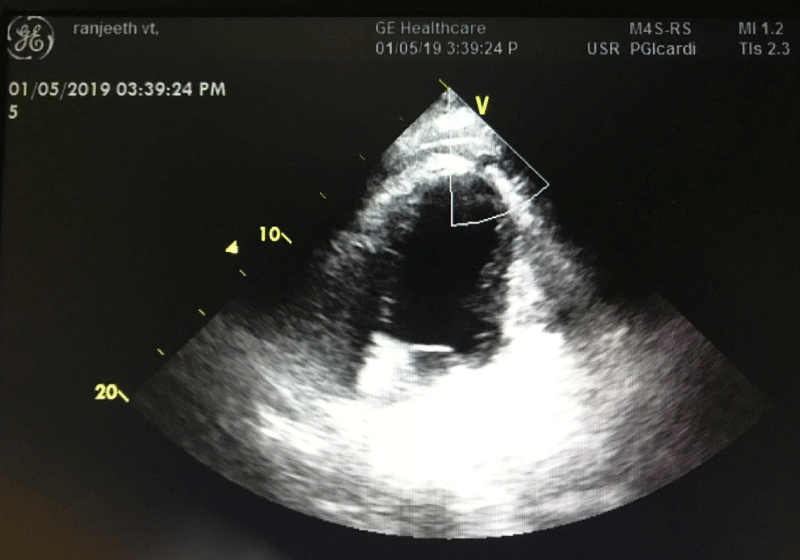
On 29 April; patient became symptomatic again with palpitations. He was found to have monomorphic VT (RBBB morphology with superior axis) [Figure 2]. He was given a bolus of 80 mg xylocard followed by infusion & 2 gm MgSO4 over 20 minutes with no response. Finally DC version was given; VT reverted to sinus rhythm temporarily only to return back in 5 minutes. DC was repeated three times but the sinus rhythm could not be maintained. He had VT refractory to AADs and DC and developed CS requiring two inotropes. The patient was sedated and electively intubated. Intra-aortic balloon pump (IABP) support was given for cardiogenic shock & temporary pacemaker (TP) was inserted. Overdrive pacing was tried but there was no return of sinus rhythm. Bilateral SGB was tried; however there was no sustained effect apart from temporary decrease in heart rate from 160 to 130/min. The CS worsened requiring four inotropes support despite on IABP. Hence a decision to put the patient on Extracorporeal membrane oxygenation (ECMO) was taken to decrease the preload and provide effective circulation. The patient was planned for catheter ablation (CA). However a review echocardiography revealed a 06 mm rent in left ventricle apex with overlying clot; there was contained rupture with mild pericardial effusion (Figures 2,3). The patient was referred to CTVS surgeon for repair of rent, aneurysmectomy and surgical cryoablation. However patient developed anuria with severe metabolic acidosis and hyperkalemia for which sustained low-efficiency dialysis (SLED) was initiated. Patient was planned for surgery after correction of metabolic parameters. However his general condition continued to deteriorate, with constant dip in sensorium and bilateral lower limb ischemia and he succumbed to his illness on 4 May 19 before the definitive surgery could be undertaken.
Discussion
VT storm or electrical storm (ES) is a state of cardiac electrical instability and refers to occurrence of three or more ventricular tachyarrhythmia’s (VT and or ventricular fibrillation (VF)) within 24 hours or VT recurring soon (within five minutes) after termination of another VT episode or sustained or no sustained VT with total ectopic beats greater than sinus beats in 24 hour period [1,2]. In patients with an implantable cardioverter-defibrillator (ICD), VT storm is defined as three or more appropriate therapies for ventricular tachyarrhythmias, including antitachycardia pacing (ATP) or shocks, within 24 hours [1,3,4].
Incidence: The incidence of VT storm varies depending upon the population studied. It is lower when ICDs are placed for primary (4%) [5], versus secondary prevention (20%) [6]. The clinical outcome of patients experiencing VT storm remains poor including increased number of non- sudden cardiac death [7,8].
Management
Step 1: Stabilize the patient hemodynamically
In our patient, we tried DC shock to revert the rhythm followed by AADs. Since patient was in cardiogenic shock (CS), patient was started on vasopressors. Mechanical circulatory support was given with IABP which has additional advantage of reducing the afterload.
Step 2: Find the triggers for electrical instability and try to reverse them. Sustained ventricular arrhythmia (VA) and/or recurrent ICD shocks may exacerbate LV systolic dysfunction culminating in CS and multi organ dysfunction [9,10]. The triggers include acute ischemia, worsening heart failure, hypokalemia, hypomagnesaemia, pro-arrhythmic drugs effects, hyperthyroidism, and infection or fever [11], Unfortunately reversible causes of VT storm account for less than 10%, and in the majority of cases no precipitating cause is identified [12].
We ruled out reversible causes of ES in our patient. He was revascularized, circulation was supported mechanically & LV afterload was reduced with IABP, electrolytes and thyroid profile was within normal limit and there was no fever. He had monomorphic VT probably scar related. TP lead was also inserted and overdrive pacing was tried but with no positive response.
Step 3: Antiarrhythmic drugs (AADs) in VT storm. Various classes of AADs are commonly used in setting of VT storm and for long term management to prevent recurrences. A recent meta-analysis found a 1.5-fold reduction of appropriate ICD interventions with AADs compared to standard medical therapy; however pooled analysis did not show a significant impact of AADs on all-cause mortality [13,14].
Beta blockers: There is increased sympathetic activity during VT storm. Hence beta blockers seem to be one of the corner stone for treatment of incessant VT. In chronic heart failure; usually beta 1 receptors get down regulated hence non selective beta blockers like propranolol have special advantage along with its ability to cross blood brain barrier and block central presynaptic adrenergic receptors [15-17].
Amiodarone: Amiodarone is one of the most commonly used drugs for control of VT/VF and its efficacy has been validated in various trials [18,19]. Greene M et al reported that amiodarone reduced the risk of recurrence of VT storm by 50% over five years follow [20]. However amiodarone especially oral should be used with caution in patients with QT prolongation. A pooled analysis by Santangeli P et al has shown increased mortality with amiodarone when comparing CA vs. AADs in VT storm [15].
Lidocaine And Mexiletene: They both act on rapid sodium channel blocker. During acute ischemia, the altered membrane potential as well as pH reduction increases the rate of drug binding; making it more efficacious in terminating the VT and hence lidocaine is recommended mostly in ischemia related ventricular arrhythmias [21,22] and it is not very effective in scar-related VTs. There is reduced burden of VAs with mexiletine when used as an adjunctive therapy to amiodarone but with a trend towards increased mortality [23].
Procainamide: Procainamide is a class IA agent, not very widely used ( & unavailable in most countries) that may be helpful to acutely terminate VAs and prevent recurrences. Gorgels et al., [24], demonstrated superiority of procainamide over lidocaine in termination of acute VT. In PROCAMIO trial [25], procainamide was found to be safe and more effective than amiodarone in treatment of tolerated VT. However the most important and dose limiting side effect is hypotension requiring drug discontinuation [25].
In our patient, we tried lidocaine which was not effective. Amiodarone was avoided in view of documented long QT interval. Our patient was already on propranolol long acting 40 mg thrice a day and mexiletene 200 mg thrice a day when he went into VT storm.
The table 1 summaries the most common AADs used in the acute and long-term management of VT, along with their side effects.
Step 4: Sedation. Sedation should be considered in all patients with incessant VT to reduce the sympathetic drive. The agents of choice include short acting benzodiazepine like midazolam and opioids like fentanyl which have minimal negative inotropic effect [26,27]. Propofol has been reported to terminate VT/VF but may cause refractory cardiogenic shock [28]. General anesthesia may be required but it increases the risk of hemodynamic decompensation [29,30].
We electively intubated our patient and sedated him with midazolam and fentanyl infusion to reduce the sympathetic drive.
Step 5: Neuraxial Modulation. Enhanced sympathetic activity plays an important role in initiation and maintenance of VT storm. Hence modulation of sympathetic output to heart may help in incessant VT. The different modalities used are unilateral/bilateral stellate ganglion blockade(SGB), surgical cardiac sympathetic denervation (CSD) and epidural anesthesia. SGB has been found effective in refractory VT [31,32,]. Most common local anesthetic agent used has been bupivacaine. In one series by Meng L et al, 80% patients survived to discharge post SGB; out of which 65% had VT storm post MI. In another meta-analysis by Fudim et al; unilateral & bilateral SGB reduced the need of defibrillation irrespective of etiology of cardiomyopathy, type of ventricular rhythm, and degree of contractile dysfunction. Surgical CSD has shown to significantly decrease the arrhythmic burden in 56% of patients refractory to AADs and CA [33]. Bilateral CSD may be considered in cases of failure of left CSD [34,35].
We tried bilateral SGB in our patient. Initially it worked to reduce the VPC burden but eventually when VT storm set in; our efforts remained futile.
Step 6: Catheter Ablation: Radiofrequency CA has not only be shown superior to medical therapy in suppressing VT storm [36-39], but has also shown to improve survival [40,41]. CA can be both endocardial or epicardial depending upon the possible origin of VT. Epicardial CA has proven to be safe and efficacious in cases where endocardial route has failed. There is a high risk of patient having acute hemodynamic decompensation during the procedure and it is especially high in patients with unstable VTs. A score known as PAINESD score is used to predict periprocedural acute hemodynamic decompensation in patients undergoing VT ablation [29] and has been validated in various studies [30] The variables included are presence of chronic obstructive pulmonary disease (COPD), age > 60 years, ischemic cardiomyopathy, New York Heart association (NYHA) class III/IV, EF < 25%, presence of VT storm and diabetes mellitus. A score ≥ 15 is associated with a risk of > 25% of acute hemodynamic decompensation during CA and warrants for a mechanical circulatory support before such a patient is undertaken for the procedure. Mechanical support not only allows prolonged mapping and ablation of inducible unstable arrhythmias but is also useful in substrate based CA when long time is required for ablation of large substrate; and irrigation during the procedure may decompensate the patient. In certain patients; LVAD and IABP may not be sufficient when ECMO may be required. In a recent study by Barrato F et al, prophylactic use of ECMO has been shown to safely complete the procedure in 92% of patients with 88% overall survival after a median follow-up of 21 months [42]. Table 2 presents the variables used in PAINESD score.
In our patient, when his VT remained refractory to all measures, a decision was made for endocardial CA. He had a very high PAINESD score of 25 and despites being on IABP, he required four vasopressors to maintain a MAP of 65mm of Hg. Apart there was apparent organ hypoperfusion with oliguria and acute kidney injury. Hence the patient was placed on ECMO ( veno arterial) to maintain circulation & reduce the preload and as a bridge to definitive treatment, i.e., CA. A veno arterial ECMO was established surgically using right internal jugular vein and right femoral vein as access ports and left femoral artery as return port with a flow rate of 3-3.2 liters /min. Activated partial thromboplastin time (aPTT) was maintained between 60-80 seconds and prophylactic antibiotic cover was given. However before he could be taken for CA; he was found to have a contained LV apical rupture (Figure 2) with mild pericardial effusion and hence CA was abandoned.
Step 7: Surgical procedures: These include aneurysmectomy of post infarction left ventricular aneurysm and extended blind cryoablation with or without perioperative mapping. Liang JJ et al., [43], had established long term survival in more than 70% patients after surgical cryoablation who presented with VT refractory to all other modalities. The other surgical option which can be tried is, as already discussed, the surgical CSD. And finally if the VT remains refractory to all modalities, cardiac transplant should be offered.
We took a cardiothoracic surgeon consult for possible aneurysmectomy, repair of left ventricle and cryoablation. But before the patient could be taken up for surgery, he went into severe metabolic acidosis with acute kidney injury requiring SLED. While the patient was being given renal replacement therapy; he started having complications peculiar to ECMO (VA) like acute limb ischemia, thrombocytopenia, progressive decrease in sensorium and sepsis as indicated by rising counts and elevated procalcitonin levels. Patient was monitored with serial echocardiography for any LV clot or cardiac tamponade. Activated partial thromboplastin time (aPTT) was kept between 60-80 seconds, platelet transfusion was given to keep platelets more than 50,000/dL and broad spectrum antibiotics covering hospital acquired gram positive & gram negative organisms were continued. However patient continued to deteriorate; and had cardiac standstill with brain death. Taking relative into confidence and after their consent, patient was declared dead on 4th April 2019 and ECMO support was removed.
This case highlights an important complication of myocardial infarction. The middle age gentleman presents late to the medical services with history of 15 days of chest pain. At admission he was documented to have cardiac arrest with polymorphic VT. The most common cause of polymorphic VT in a post MI patient is ischemia; hence he was taken for revascularization. Post revascularization; he continued to have recurrent episodes of monomorphic VT (right bundle branch block [RBBB] morphology with superior axis) with possible origin from left ventricular apex. Despite all measures, we could not save our patient. We sort of used all measures in our armamentarium; other than surgical options. This case emphasizes how challenging the management of VT storm can be especially in presence of mechanical complication.
The aim of this case report was to discuss the various available treatment options available for VT storm and practical problems faced during management of such a patient.
- Al-Khatib SM, Stevenson WG, Ackerman MJ (2018) 2017 AHA/ACC/HRS Guideline for Management of Patients With Ventricular Arrhythmias and the Prevention of Sudden Cardiac Death: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. Heart Rhythm 15: e190-e252. Link: http://bit.ly/2JX2rl8
- Kowey PR, Levine JH, Herre JM (1995) Randomized, double-blind comparison of intravenous amiodarone and bretylium in the treatment of patients with recurrent, hemodynamically destabilizing ventricular tachycardia or fibrillation. The Intravenous Amiodarone Multicenter Investigators Group. Circulation 92: 3255-3263. Link: http://bit.ly/2Opbtvq
- Israel CW, Barold SS (2007) Electrical storm in patients with an implanted defibrillator: a matter of definition. Ann Noninvasive Electrocardiol 12: 375-382. Link: http://bit.ly/2Mg8Dq1
- Haegeli LM, Della Bella P, Brunckhorst CB (2016) Management of a Patient With Electrical Storm: Role of Epicardial Catheter Ablation. Circulation 133: 672-676. Link: http://bit.ly/2ygla4I
- Sesselberg HW, Moss AJ, McNitt S, Zareba W, Daubert JP, et al. (2007) Ventricular arrhythmia storms in postinfarction patients with implantable defibrillators for primary prevention indications: a MADIT-II substudy. Heart Rhythm 4: 1395-1402. Link: http://bit.ly/2GuNzIs
- Exner DV, Pinski SL, Wyse DG, Renfroe EG, Follmann D, et al. (2001) Electrical storm presages nonsudden death: the antiarrhythmics versus implantable defibrillators (AVID) trial. Circulation 103: 2066-2071. Link: http://bit.ly/2JX7HW1
- Gatzoulis KA, Andrikopoulos GK, Apostolopoulos T, Sotiropoulos E, Zervopoulos G, et al. (2005) Electrical storm is an independent predictor of adverse long-term outcome in the era of implantable defibrillator therapy. Europace 7: 184-192. Link: http://bit.ly/2LIPu0t
- Credner SC, Klingenheben T, Mauss O, Sticherling C, Hohnloser SH (1998) Electrical storm in patients with transvenous implantable cardioverter-defibrillators: incidence, management and prognostic implications. J Am Coll Cardiol 32: 1909-1915. Link: http://bit.ly/2GyXVqO
- Zaugg CE, Wu ST, Barbosa V, Buser PT, Wikman-Coffelt J, et al. (1998) Ventricular fibrillation-induced intracellular Ca2+ overload causes failed electrical defibrillation and post-shock reinitiation of fibrillation. J Mol Cell Cardiol 30: 2183-2192. Link: http://bit.ly/2YikK8o
- Joglar JA, Kessler DJ, Welch PJ, Keffer JH, Jessen ME, et al. (1999) Effects of repeated electrical defibrillations on cardiac troponin I levels. Am J Cardiol 83: 270-272. Link: http://bit.ly/2MkAVPZ
- European Heart Rhythm Association, Heart Rhythm Society, Zipes DP, Camm AJ, Borggrefe M, et al. (2006) ACC/AHA/ESC 2006 guidelines for management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: a report of the American College of Cardiology/American Heart Association Task Force and the European Society of Cardiology Committee for Practice Guidelines (Writing Committee to Develop Guidelines for Management of Patients with Ventricular Arrhythmias and the Prevention of Sudden Cardiac Death). J Am Coll Cardiol 48: e247-346. Link: http://bit.ly/2YqBnD4
- Hohnloser SH, Al-Khalidi HR, Pratt CM, Brum JM, Tatla DS, et al. (2006) Electrical storm in patients with an implantable defibrillator: incidence, features, and preventive therapy: insights from a randomized trial. Eur Heart J 27: 3027-3032. Link: http://bit.ly/2Y6tG5C
- Santangeli P, Muser D, Maeda S, Filtz A, Zado ES, et al. (2016) Comparative effectiveness of antiarrhythmic drugs and catheter ablation for the prevention of recurrent ventricular tachycardia in patients with implantable cardioverter-defibrillators: A systematic review and metaanalysis of randomized controlled trials. Heart Rhythm 13: 1552-1559. Link: http://bit.ly/2JWkaJp
- Stanton MS, Prystowsky EN, Fineberg NS, Miles WM, Zipes DP, et al. (1989) Arrhythmogenic effects of antiarrhythmic drugs: a study of 506 patients treated for ventricular tachycardia or fibrillation. J Am Coll Cardiol 14: 209-215. Link: http://bit.ly/2LJvx9I
- Nademanee K, Taylor R, Bailey WE, Rieders DE, Kosar EM (2000) Treating electrical storm: sympathetic blockade versus advanced cardiac life support-guided therapy. Circulation 102: 742-747. Link: http://bit.ly/2GyYYXR
- Bristow MR, Ginsburg R, Umans V, Fowler M, Minobe W, et al. (1986) Beta 1- and beta 2-adrenergic-receptor subpopulations in nonfailing and failing human ventricular myocardium: coupling of both receptor subtypes to muscle contraction and selective beta 1-receptor downregulation in heart failure. Circ Res 59: 297-309. Link: http://bit.ly/2Ym2FpV
- Tsagalou EP, Kanakakis J, Rokas S, Anastasiou-Nana MI (2005) Suppression by propranolol and amiodarone of an electrical storm refractory to metoprolol and amiodarone. Int J Cardiol 99: 341-342. Link: http://bit.ly/2YmEqbk
- Kowey PR, Crijns HJ, Aliot EM, Capucci A, Kulakowski P, et al. (2011) Efficacy and safety of celivarone, with amiodarone as calibrator, in patients with an implantable cardioverter-defibrillator for prevention of implantable cardioverter-defibrillator interventions or death: the ALPHEE study. Circulation 124: 2649-2660. Link: http://bit.ly/2Y6tKCo
- Connolly SJ, Dorian P, Roberts RS, Gent M, Bailin S, et al. (2006) Comparison of betablockers, amiodarone plus beta-blockers, or sotalol for prevention of shocks from implantable cardioverter defibrillators: the OPTIC Study: a randomized trial. JAMA 295: 165-171. Link: http://bit.ly/32Tpzsd
- Greene M, Newman D, Geist M, Paquette M, Heng D, et al. (2000) Is electrical storm in ICD patients the sign of a dying heart? Outcome of patients with clusters of ventricular tachyarrhythmias. Europace 2: 263-269. Link: http://bit.ly/2K7P4gP
- MacMahon S, Collins R, Peto R, Koster RW, Yusuf S (1988) Effects of prophylactic lidocaine in suspected acute myocardial infarction. An overview of results from the randomized, controlled trials. JAMA 260: 1910-1916. Link: http://bit.ly/2YpAdDu
- European Heart Rhythm Association, Heart Rhythm Society, Zipes DP, Camm AJ, Borggrefe M, et al. (2006) ACC/ AHA/ESC 2006 guidelines for management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: a report of the American College of Cardiology/American Heart Association Task Force and the European Society of Cardiology Committee for Practice Guidelines (Writing Committee to Develop Guidelines for Management of Patients With Ventricular Arrhythmias and the Prevention of Sudden Cardiac Death). J Am Coll Cardiol 48: e247-e346. Link: http://bit.ly/2YqBnD4
- Gao D, Van Herendael H, Alshengeiti L, Dorian P, Mangat I, et al. (2013) Mexiletine as an adjunctive therapy to amiodarone reduces the frequency of ventricular tachyarrhythmia events in patients with an implantable defibrillator. J Cardiovasc Pharmacol 62: 199-204. Link: http://bit.ly/2OmwAyo
- Gorgels AP, van den Dool A, Hofs A, Mulleneers R, Smeets JL, et al. (1996) Comparison of procainamide and lidocaine in terminating sustained monomorphic ventricular tachycardia. Am J Cardiol 78: 43-46. Link: http://bit.ly/2Yqq51z
- Ortiz M, Martín A, Arribas F, Coll-Vinent B, Del Arco C, et al. (2016) PROCAMIO Study Investigators. Randomized comparison of intravenous procainamide vs. intravenous amiodarone for the acute treatment of tolerated wide QRS tachycardia: the PROCAMIO study. Eur Heart J 38: 1329-1335. Link: http://bit.ly/2JWkPdR
- Mandel JE, Hutchinson MD, Marchlinski FE (2011) Remifentanilmidazolam sedation provides hemodynamic stability and comfort during epicardial ablation of ventricular tachycardia. J Cardiovasc Electrophysiol 22: 464-466. Link: http://bit.ly/2MnNR85
- Ogletree ML, Sprung J, Moravec CS (2005) Effects of remifentanil on the contractility of failing human heart muscle. J Cardiothorac Vasc Anesth 19: 763-767. Link: http://bit.ly/30XBtzp
- Mulpuru SK, Patel DV, Wilbur SL, Vasavada BC, Furqan T (2008) Electrical storm and termination with propofol therapy: a case report. Int J Cardiol 128: e6-e8. Link: http://bit.ly/2KaQLKF
- Santangeli P, Muser D, Zado ES, Magnani S, Khetpal S, et al. (2015) Acute hemodynamic decompensation during catheter ablation of scar-related ventricular tachycardia: incidence, predictors, and impact on mortality. Circ Arrhythm Electrophysiol 8: 68–75. Link: http://bit.ly/2yjBwcs
- Mathuria N, Wu G, Rojas-Delgado F, Shuraih M, Razavi M, et al. (2016) Outcomes of pre-emptive and rescue use of percutaneous left ventricular assist device in patients with structural heart disease undergoing catheter ablation of ventricular tachycardia. J Interv Card Electrophysiol 48: 27-34. Link: http://bit.ly/2Y7d5i7
- Meng L, Tseng CH, Shivkumar K, Ajijola O (2017) Efficacy of Stellate Ganglion Blockade in Managing Electrical Storm: A Systematic Review. JACC Clin Electrophysiol 3: 942-949. Link: http://bit.ly/2ZdWvcB
- Fudim M, Boortz-Marx R, Ganesh A (2017) Stellate ganglion blockade for the treatment of refractory ventricular arrhythmias: A systematic review and meta-analysis. J Cardiovasc Electrophysiol 28: 1460-1467. Link: http://bit.ly/2K0VqhS
- Bourke T, Vaseghi M, Michowitz Y, Sankhla V, Shah M, et al. (2010) Neuraxial modulation for refractory ventricular arrhythmias: value of thoracic epidural anesthesia and surgical left cardiac sympathetic denervation. Circulation 121: 2255-2262. Link: http://bit.ly/30ZP09W
- Ajijola OA, Lellouche N, Bourke T, Tung R, Ahn S, et al (2012) Bilateral cardiac sympathetic denervation for the management of electrical storm. J Am Coll Cardiol 59: 91-92. Link: http://bit.ly/310JSSN
- Vaseghi M, Gima J, Kanaan C, Ajijola OA, Marmureanu A, et al. (2014) Cardiac sympathetic denervation in patients with refractory ventricular arrhythmias or electrical storm: intermediate and long-term follow-up. Heart Rhythm 11: 360-366. Link: http://bit.ly/2OimoXM
- Sapp JL, Wells GA, Parkash R, Stevenson WG, Blier L, et al. (2016) Ventricular Tachycardia Ablation versus Escalation of Antiarrhythmic Drugs. N Engl J Med 375: 111-121. Link: http://bit.ly/2ZikSGc
- Liang JJ, Muser D, Santangeli P (2017) Ventricular Tachycardia Ablation Clinical Trials. Card Electrophysiol Clin 9: 153-165. Link: http://bit.ly/2MhHqDb
- Carbucicchio C, Santamaria M, Trevisi N, Maccabelli G, Giraldi F, et al. (2008) Catheter ablation for the treatment of electrical storm in patients with implantable cardioverter-defibrillators: short- and long-term outcomes in a prospective single-center study. Circulation 117: 462-469. Link: http://bit.ly/2SG6IMA
- Di Biase L, Santangeli P, Burkhardt DJ, Bai R, Mohanty P, et al. (2012) Endo-epicardial homogenization of the scar versus limited substrate ablation for the treatment of electrical storms in patients with ischemic cardiomyopathy. J Am Coll Cardiol 60: 132-141. Link: http://bit.ly/2YsHOW7
- Tung R, Vaseghi M, Frankel DS, Vergara P, Di Biase L, et al. (2015) Freedom from recurrent ventricular tachycardia after catheter ablation is associated with improved survival in patients with structural heart disease: An International VT Ablation Center Collaborative Group study. Heart Rhythm 12: 1997-2007. Link: http://bit.ly/2LJ7BTW
- Muser D, Santangeli P, Castro SA, Pathak RK, Liang JJ, et al. (2016) Long-Term Outcome After Catheter Ablation of Ventricular Tachycardia in Patients With Nonischemic Dilated Cardiomyopathy. Circ Arrhythm Electrophysiol 9: pii e004328. Link: http://bit.ly/2YqBTRw
- Baratto F, Pappalardo F, Oloriz T, Bisceglia C, Vergara P, et al. (2016) Extracorporeal Membrane Oxygenation for Hemodynamic Support of Ventricular Tachycardia Ablation. Circ Arrhythm Electrophysiol : pii: e004492. Link: http://bit.ly/2SLOtFt
- Liang JJ, Betensky BP, Muser D, Zado ES, Anter E, et al. (2017) Long term outcome of surgical cryoablation for refractory ventricular tachycardia in patients with non-ischemic cardiomyopathy. Europace 20: e30-e41. Link: http://bit.ly/2MkvnoW