Journal of Cardiovascular Medicine and Cardiology
Cardiac Myxomas - Symptomology, Investigations and Surgical Treatment - Single Centre Experience of Fifteen Years at Grant Medical College, Mumbai, Maharashtra
Suraj Wasudeo Nagre1* and Krishnarao N Bhosle2
2Professor and Head of Department CVTS, Grant Medical College, Mumbai, India
Cite this as
Suraj WN, Krishnarao NB (2018) Cardiac Myxomas - Symptomology, Investigations and Surgical Treatment - Single Centre Experience of Fifteen Years at Grant Medical College, Mumbai, Maharashtra. J Cardiovasc Med Cardiol 5(2): 013-015. DOI: 10.17352/2455-2976.000062Introduction: The aim of study is to describe the clinical symptoms, investigation findings and surgical treatment of cardiac myxomas.
Method: From May 2002 to May 2017, 50 patients of primary or recurrent intracardiac myxoma underwent surgical excision at our institute. Their age ranged from 25 years to 55 years. Out of which 20 males and 30 females. Commonest presenting symptoms are dyspnea and palpitation. 35 Left atrial, 13 Right atrial, one right ventricular and one left ventricular myxoma. The diagnosis was done by transthoracic and transesophageal echocardiography. The complete wide excision with margin of 3-5 mm normal surrounding tissue was the main principle of surgery. Right atriotomy, right ventricular and left ventricular surgical approach was used. Post-operative echocardiogram was done in all patients before discharge. Maximal follow-up of five years and minimum follow-up of 6 months was done after surgery.
Results: No mortality. On followup after five years of surgery, all patients were in NYHA class 1 and their echocardiography showed good ventricular function with normal pulmonary artery pressure with patch in situ. One of patient right atrial myxoma developed left atrial myxoma after five years of first surgery that also excised.
Conclusion: We recommend right atriotomy approach for both right and left atrial myxomas. Right ventricle approach for right ventricular myxoma and left ventricular for left ventricular myxoma. Biatrial approach in large and unusually located left atrial myxoma. To prevent recurrence the surgical excision must include a substantial portion of normal surrounding tissue near the base of implantation. With proper surgical technique no mortality and recurrence with complete recovery.
Introduction
Over 72% of primary cardiac tumors are benign. In adults, the majority of benign lesions are myx-omas. Cardiac myxoma are uncommon tumours found in 0.5 million population per year with left atrium being the predominant site. They vary widely in size, and very little is known about their growth rate. The reported growth rates of left atrial myxomas from several published case reports appears to vary from no growth, to between 1.3 to 6.9 mm/month in diameter within patients with established myxoma who have not undergone surgery. The prevalence of cardiac tumors at autopsy ranges from 0.001% to 0.3%, more than 50% of be-nign cardiac tumors are myxomas. In 7%, it has genetic origin and rises as a component of a heritable disorder with some clinical manifestations. The aim of study is to describe the clinical symptoms, investigation findings and surgical treatment of cardiac myxomas in our institute Grant Medical College, Mumbai.
Material and Method
From May 2002 to May 2017, 50 patients of primary or recurrent intracardiac myxoma underwent surgical excision at our institute. Their age ranged from 25 years to 55 years. Out of which 20 males and 30 females. Commonest presenting symptoms are dyspnea and palpitation. Common sites found are Left atrial 35, Right atrial 13, one right ventricular and one left ventricular myxoma (Table 1).
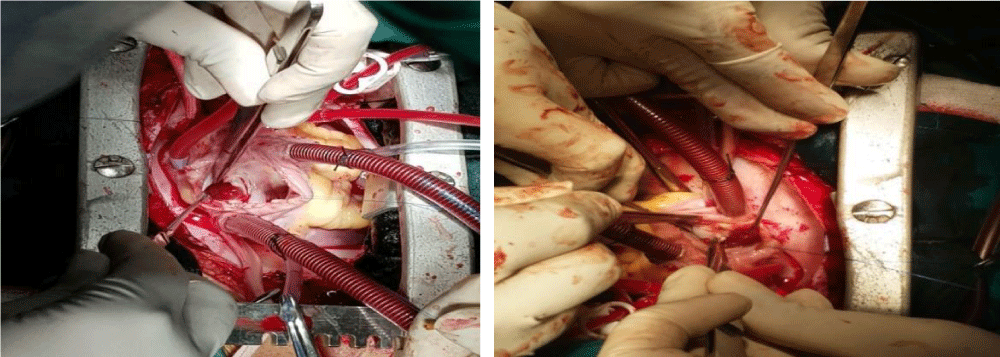
The diagnosis was done by transthoracic and transesophageal echocardiography. The complete wide excision with margin of 3-5 mm normal surrounding tissue under GA and CPB with no touch technique with closure of defect by autologous pericardial patch was the main principle of surgery (Figure 1).
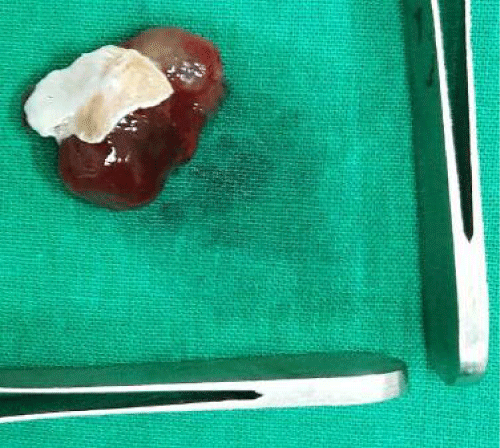
We has used right atriotomy approach for both right and left atrial myxomas, right ventricle approach for right ventricular myxoma and left ventricular for left ventricular myxoma. Biatrial approach was used only in one patient with large and unusually located left atrial myxoma. Defect created by excision closed with pericardial patch. Excised specimen was sent for histopathological examination and confirmation of diagnosis (Figure 2).
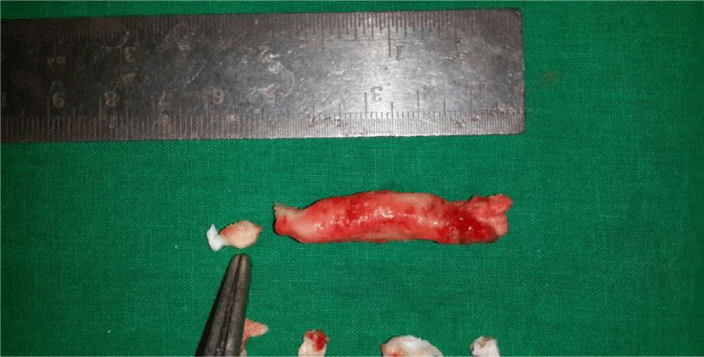
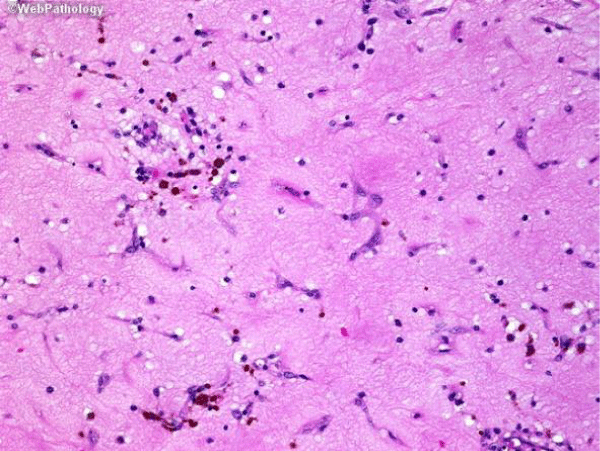
One of the patient was known case of ischemic heart disease preopt diagnosed with left ventrical free clot (Figure 3), but on histopathological examination diagnosed as left ventricular myxoma (Figure 4).
Post-operative echocardiogram was done in all patients before discharge. Maximal follow-up of five years and minimum follow-up of 6 months was done after surgery.
Results
No short and long term mortality in any of operated cases. On follow-up after five years of surgery, all patients were in NYHA class 1 and their echocardiography showed good ventricular function with normal pulmonary artery pressure with patch in situ. One of patient right atrial myxoma developed left atrial myxoma after five years of first surgery that also excised. So it is not recurrence as site of origin was different from the previous site of myxoma.
Discussion
Atrial myxoma is the most common benign tumor of the heart and has a greater predilection to the left atrium. Embolic phenomena is thought to be caused by either tumor detachment or clot embolization. Patients can present with dyspnea on exertion, palpitations or congestive heart failure. Autoimmune features include: constitutional symptoms, fatigue, fever, my-algia, arthalgia, muscle weakness, rash, and weight loss [1]. Activation of pro-inflammatory cytokines such as: IL-6 and Tumor Necrosis Factor alpha (TNFa) have been implicated. Often thrombolysis is given to patients presenting with stroke prior to diagnosing atrial myxoma. Although, randomized clinical data is lacking, managing this challenging scenario has resulted in variable results including: clinical improvement, persistent neurological deficits or death [2].
The origin can be explained through different theories. Myxomas are currently thought to originate from entrapped embryonic foregut and hence they are derived from multipotentmesenchymal cells capable of both neural and epithelial differentiation. Histologically, these tumors are composed of scattered cells within a mucopolysaccharidestroma [3]. Myxomas produce vascular endothelial growth factor (VEGF), which probably contributes to the induction of angiogenesis and the early stages of tumor growth.
On a macroscopic level, typical myxomas are pedunculated and gelatinous in consistency; the surface may be smooth, villous, or friable. Tumors vary widely in size, ranging from 1 to 15 cm in diameter, and weighing between 15 and 180gm. About 35 percent of myxomas are friable or villous, and these tend to present with emboli. Larger tumors are more likely to have a smooth surface and to be associated with cardiovascular symptoms.
Systemic embolisation and sudden death are common, which warrant an early surgical intervention [4]. Although quite rare, left atrial myxomas account for 80% of all cardiac tumors. Diagnosis is often difficult due to the wide array of presenting symptoms. Atrial myxomas are associated with systemic embolization in 30 to 40% of cases. These intracardiac growths may mas-querade as mitral stenosis, infective endocarditis, and collagen vascular disease, which can further impede accurate diagnosis. Constitutional symptoms (e.g., fever, weight loss) are seen in around 30 percent of patients. Laboratory abnormalities (e.g., anemia and elevations in the erythrocyte sedimentation rate, C-reactive protein, or globulin level) are present in 35 percent, usually those with systemic symptoms.
Common symptoms and signs of dyspnea, orthopnea, paroxysmal nocturnal dyspnea, pulmonary edema, cough, hemoptysis, edema, and fatigue lead to a wide differential diagnoses, making it critical for clinicians to be consider atrial mxyoma. The goals of the initial evaluation are to ascertain whether or not a cardiac tumor is present, the location of the lesion within the heart and, to the extent possible, whether a tumor is benign or malignant. This information is vital in planning further evaluation and management. Echocardiography, cardiac MRI, and ultra-fast CT provide complementary information to address these questions [5].
Echocardiography is widely available and provides a simple, noninvasive technique for the initial evaluation. Echocardiography images both the myocardium and the cardiac chambers can usually identify the presence of a mass. In addition, echocardiography may provide information about any obstruction to the circulation, as well as the likelihood that the tumor could be a source of emboli [6].
Although Transthoracic Echocardiography (TTE) is simple and usually can identify a tumor, Transesophageal Echocardiogra-phy (TEE) may be more informative. The superior diagnostic utility of TEE is due to the proximity of the esophagus to the heart, the lack of intervening lung and bone, and the ability to use high-frequency imaging transducers that afford superior spatial resolution. In our patient, the TTE was sufficient in confirming the diagnosis.
Treatment and prognosis: Once a presumptive diagnosis of myxoma has been made on imaging studies, prompt resection is required because of the risk of embolization or cardiovascular complications, including sudden death. The results of surgical resection are generally very good, with most series reporting an operative mortality rate under 5 percent. Cardiac transplantation has been reported for other tumors and might be considered for multiple, recurrent atrial myxomas [7].
Postoperative recovery is generally rapid. However, atrial arrhythmias or atrioventricular conduction abnormalities were present postoperatively in 26 percent of patients in one series. In addition, patients are at risk for recurrence of the myxoma or the development of additional lesions. In one large series, 5 percent developed recurrent myxoma, suggesting the need for careful follow-up. Development of a second primary myxoma may be more common in patients with a family history of myxoma as described by Nagre et al in one study [8].
Rapidly growing myxoma may be mistaken for thrombus, and may require urgent surgical excision to reduce the risk of associated complications such as thrombo-embolic events, sudden cardiac death and removal of a possibly malignant tumor. The potential for rapid growth should be considered if there is a plan to delay surgery [9]. Our experience suggests that surgery for atrial myxoma is safe and simple by precautions to protect from embolisation like minimal handling of heart before going on bypass and use of heamofilter during cardiopulmonary bypass. Although atrial myxomas are usually benign or asymptomatic, there is the possibility of diastolic embolization, conduction alterations and disturbances, and lethal valve obstructions occurring [10]. Since surgical excision has been reported to alleviate symptoms associated with cardiac myxomas, early identification and removal is preferable.
Conclusion
Early surgical intervention after diagnosis can significantly reduce the possibility of sudden death and other embolic complications. We recommend right atriotomy approach for both right and left atrial myxomas. Right ventricle approach for right ventricular myxoma and left ventricular for left ventricular myxoma. Biatrial approach was required only in large and unusually located left atrial myxoma. To prevent recurrence the surgical excision must include a substantial portion of normal surrounding tissue near the base of implantation and closure of defect with pericardial patch. With proper surgical technique no mortality and recurrence with complete recovery.
- Nagre SW (2015) Left atrial myxoma excision in indian journal of research. Paripex Medical Science Research Paper 4: 263-265.
- Nagre SW (2016) Atrial Septal Defect: Management Approach in Children. Ann Woman Child Health 2: 3-4. Link: https://goo.gl/gZH1po
- Nagre SW (2015) Left atrial myxoma excision in international journal of scientific research. Paripex Medical Science Research Paper 4: 71-73.
- Nagre SW (2017) Mobile left atrial mass-clot or left atrial myxoma. J Cardiovasc Dis Res 8: 31-34. Link: https://goo.gl/tsu7Je
- Nagre SW (2016) Management of Patent Ductous Arteriosus - Short Review. Annals of Woman and Child Health 2. Link: https://goo.gl/SPM9LX
- Nagre SW (2016) Editorial article in Journal of Medical Oncology and Therapeutics 1.
- Nagre SW, Nagre MS (2015) Observational Study of Surgical Closure of Ostium Primum Atrial Septal Defect in Thirty Paediatric Patients. AWCH 1: 1-4. Link: https://goo.gl/TaK7Do
- Nagre SW (2016) Mobile left atrial mass-clot or left atrial myxoma. J Med Oncl Ther 1: 94-96. Link: https://goo.gl/Mz6Z2V
- Nagre SW (2017) Atrial Septal Defect - Forgotten Treatment Guidelines. Acute Chronic Diss 1: e101. Link: https://goo.gl/7W4g8C
- Nagre SW (2015) Surgical removal of embolised atrial septal defect device from pulmonary artery. J Thorac Cardiovasc Surg 150: e55-57. Link: https://goo.gl/LV77ar